Abstract
The effects of body mass index on the diagnostic accuracy of primary aldosteronism (PA) are inconsistent and yet important considering the high prevalence and frequent co‐occurrence of obesity and hypertension. The current study included 59 adult patients who underwent a stepwise evaluation for PA, using aldosterone to renin ratio for case detection and plasma aldosterone concentration after saline suppression test and/or 24‐hour urinary aldosterone after oral sodium loading for case confirmation. Body mass index had a quadratic (U‐shaped) correlation with plasma aldosterone concentration, plasma renin activity, aldosterone to renin ratio, and plasma aldosterone concentration after saline suppression test. Among patients with a body mass index ≥30 kg/m2, the aldosterone to renin ratio yielded lower case detection accuracy of PA. We conclude that obesity results in a nonlinear correlation with plasma aldosterone concentration, plasma renin activity, and aldosterone to renin ratio, which affects the accuracy of case detection for PA. Patients with a body mass index ≥30 kg/m2 are less accurately identified as having PA when saline suppression and/or oral salt loading tests are used for case confirmation.
1. INTRODUCTION
Primary aldosteronism (PA) is the leading cause of endocrine hypertension and encompasses a group of diseases in which aldosterone is autonomously secreted from the adrenal glands.1 The most common cause of PA is bilateral adrenocortical hyperplasia, followed by aldosterone‐producing adenomas. Current studies estimate that PA is the underlying etiology in approximately 10% of patients with hypertension.2 Identifying patients with PA is clinically important given their increased cardiovascular risk and the potential for surgical and other treatment.3
The high prevalence of PA and its deleterious effects3 on the cardiovascular system requires a diagnostic algorithm that enables accurate case detection. The 2016 Endocrine Society Guidelines1 recognize PA as a major public health issue given its high prevalence, and suggests screening approximately 50% of hypertensive patients.4 An established case detection test for PA is the plasma aldosterone concentration (PAC) to plasma renin activity (PRA) ratio (ARR). Significant variability in cutoff values exist mainly because of a lack of uniformity in diagnostic protocols, cohort selection, assay methods, disease definition, and individual factors, including medication use, age, sex, and presence of renal disease.1 Suppression tests using either saline infusion (SST), oral sodium loading, fludrocortisone, or captopril challenge tests are used for case confirmation with varying sensitivity and specificity.
In the United States, 34.9% of adults are obese.5 Although increased aldosterone levels are one of the suggested drivers for obesity‐related hypertension,6 the exact mechanisms remain elusive.7 Previous studies have shown that body mass index (BMI) correlates with PAC and urinary aldosterone (UA) excretion in normotensive healthy persons and women,8 and an increased ARR has been described in obese hypertensive women.9 However, the impact of BMI on PAC, ARR, and the accuracy of PA case detection has yielded inconsistent results.8, 10, 11, 12, 13 Thus, we sought to determine the impact of obesity on the case detection of PA, which has important implications for the interpretation of ARR in the evaluation of individuals with hypertension.
2. PATIENTS AND METHODS
2.1. Clinical protocol
A prospective analysis from 2002 to 2015 of 59 patients with PA was performed. Patients were referred for clinical evaluation based on the following categories: adrenocortical incidentaloma or bilateral adrenocortical hyperplasia (49.2%), hypokalemic hypertension (32.2%), and resistant hypertension (18.6%, Figure 1). Patients with suspected Cushing syndrome or co‐secretors (secretion of both aldosterone and cortisol) were excluded from the analysis. All patients gave written informed consent and were recruited under protocol 00‐CH‐0160 (clinical trial number NCT00005927) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Per protocol, all patients with or without elevated ARR underwent confirmatory testing with SST and oral salt loading as outlined later. The protocol was not designed to include the fludrocortisone or captopril challenge tests for case confirmation. Automated office blood pressure (BP) measurement was performed in all patients throughout their evaluation.
Figure 1.
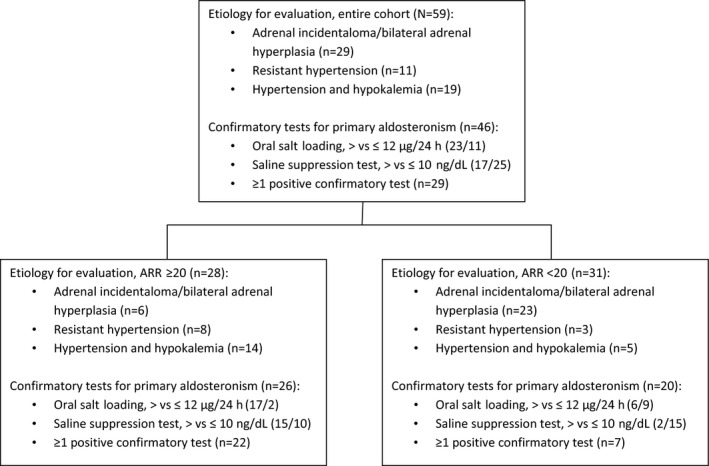
Flowchart of the distribution of our cohort per etiology and confirmatory tests for primary aldosteronism. ARR indicates aldosterone to renin ratio. Resistant hypertension is defined as blood pressure above goal in spite of full doses of at least 3 antihypertensive medications, including a diuretic
2.2. Medical treatment
As outlined by the Clinical Practice Guidelines of the Endocrine Society on PA,1 medications that may lead to false‐positive or false‐negative ARR results were all discontinued at least 2 weeks prior to the initial biochemical evaluation, and treatment with a mineralocorticoid receptor antagonist, such as spironolactone, was discontinued at least 4 weeks prior to testing. Antihypertensive therapy with minimal effect on ARR was achieved with one or more of the following oral agents: α‐blocker (doxazosin or terazosin), calcium channel blocker (verapamil), and/or vasodilator (hydralazine). Patients taking mineralocorticoid receptor antagonists, angiotensin‐converting enzyme inhibitors, or angiotensin II receptor blockers during the evaluation were excluded from the current analysis. Potassium was normalized in all patients and liberal salt intake prior to biochemical evaluation was encouraged.
2.3. Biochemical evaluation
Patients underwent a stepwise diagnostic approach for PA diagnosis, as previously described.1, 14 PAC (ng/dL) and 24‐hour UA (μg/24 h) were measured using liquid chromatography–tandem mass spectrometry (Mayo Medical Laboratories). PRA (ng/mL∙h) was calculated by incubating its substrate, angiotensinogen, and measuring the product angiotensin I by tandem mass spectrometry (Mayo Medical Laboratories). ARR was reported in conventional units (ng/dL per ng/mL∙h), with an adopted cutoff value of ≥20 for a positive case detection for PA.1 All patients underwent SST and oral salt‐loading tests and 24‐hour UA for confirmation of PA.1 SST was performed in the morning with a continuous infusion of 2 L of 0.9% normal saline over 4 hours and PAC measured at baseline and hourly. Postinfusion PACs (suppressed PAC, sPAC) >10 ng/dL were considered confirmatory for PA. During the oral salt‐loading test, patients were given 2 g of sodium tablets three times daily for 3 days and urinary sodium and aldosterone were measured in a 24‐hour urine collection from the morning of day 3 until the morning of day 4. UA (suppressed UA) >12 μg/24 h along with a urine sodium >200 nmol/24 h confirmed the diagnosis of PA.
2.4. Statistical analysis
Statistical calculations were performed with SPSS 20.0 software (SPSS Inc). Results are expressed as mean±standard deviation unless otherwise indicated. BMI and suppressed UA were normally distributed, whereas PAC, PRA, ARR, and sPAC were all logarithmically transformed to induce approximate normality. For group comparisons, the independent Student t test or one‐way analysis of variance were used to analyze differences in numerical variables, and the chi‐square test was employed to analyze differences in categorical variables. The Pearson product was used for analysis of correlations between variables. Since the associations between BMI and PAC and other variables showed a U‐shaped correlation and not a linear relationship, we performed a statistical analysis for quadratic correlation; the F test was conducted to estimate the superiority of quadratic correlation over linear correlation. The r correlation coefficient changes between the linear and quadratic correlations and the P values for these changes are presented. The P value for significance was set at <.05.
3. RESULTS
3.1. Descriptive characteristics
The current analysis included 59 patients, with a mean age of 49.3±16.0 years and mean BMI of 32.6±6.8 kg/m2; 39 (66.1%) were women. The patients were divided into five subgroups according to their BMI, selected based on BMI cutoff values used by the Endocrine Society Guidelines for pharmaceutical management of obesity15 (characteristics detailed in Table 1): 18 to 24.9 kg/m2 (n=7), 25 to 29.9 kg/m2 (n=12), 30 to 34.9 kg/m2 (n=22), 35 to 39.9 kg/m2 (n=9), and ≥40 kg/m2 (n=9). Seventeen patients had a negative confirmatory test for PA (Figure 1).
Table 1.
Study population characteristics according to BMI categories
| Entire cohort | BMI category | P value | |||||
|---|---|---|---|---|---|---|---|
| 18.0–24.9 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | ≥40 | |||
| No., % | 59 (100) | 7 (11.9) | 12 (20.3) | 22 (37.3) | 9 (15.3) | 9 (15.3) | |
| BMI, kg/m2 | 32.6±6.8 | 21.6±2.4 | 26.7±1.3 | 32.9±1.2 | 37.5±1.8 | 43.4±2.5 | <.001 |
| Age, y | 49.3±16.0 | 35.6±14.1 | 50.1±8.9 | 52.1±11.9 | 51.6±12.5 | 50.0±8.5 | .02 |
| Women, No. (%) | 39 (66.1) | 7 (100) | 7 (58.3) | 11 (50.0) | 5 (55.6) | 9 (100) | .02 |
| Ethnicity, No. (%) | |||||||
| White | 37 (62.7) | 3 (42.9) | 9 (75.0) | 13 (59.1) | 6 (66.7) | 6 (66.7) | NS |
| Black | 14 (23.7) | 0 | 2 (16.6) | 8 (36.4) | 1 (11.1) | 3 (33.3) | |
| Hispanic | 3 (5.1) | 1 (14.3) | 1 (8.4) | 0 | 1 (11.1) | 0 | |
| Asian | 3 (5.1) | 2 (28.6) | 0 | 1 (4.5) | 0 | 0 | |
| Other or unknown | 2 (3.4) | 1 (14.3) | 0 | 0 | 1 (11.1) | 0 | |
| SBP, mm Hg | 137±17 | 137±15 | 132±18 | 142±19 | 134±15 | 132±16 | NS |
| ≥140 mm Hg, No. (%) | 27 (45.8) | 3 (42.9) | 5 (41.7) | 12 (54.5) | 4 (44.4) | 3 (33.3) | NS |
| DBP, mm Hg | 79±12 | 89±9 | 79±11 | 79±15 | 77±11 | 76±10 | NS |
| ≥90 mm Hg, No. (%) | 11 (18.6) | 3 (42.9) | 1 (8.3) | 5 (22.7) | 1 (11.1) | 1 (11.1) | NS |
| PAC, ng/dL | 22.8±19.2 | 56.9±28.9 | 18.3±9.8 | 18.2±12.6 | 18.4±12.5 | 17.9±13.4 | .007a |
| ≥15 ng/dL, No. (%) | 35 (59.3) | 7 (100) | 5 (45.5) | 13 (59.1) | 5 (55.6) | 5 (55.6) | NS |
| PRA, ng/mL/h | 2.5±4.9 | 7.5±11.2 | 2.6±3.6 | 1.8±3.8 | 0.9±0.6 | 1.8±1.4 | NS |
| ARRb | 26.3±27.3 | 54.4±50.4 | 19.6+18.4 | 0.8±22.9 | 29.1±22.8 | 14.0±14.7 | NS |
| ARR ≥20, No. (%) | 28 (47.5) | 4 (57.1) | 5 (41.7) | 12 (54.5) | 6 (66.7) | 1 (11.1) | NS |
| ARR ≥30, No. (%) | 20 (33.9) | 4 (57.1) | 3 (25.0) | 7 (31.8) | 5 (55.6) | 1 (11.1) | NS |
Kruskal‐Wallis test.
Aldosterone to renin ratio (ARR) in conventional units (ng/dL per ng/mL∙h).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; NS, not significant; PAC, plasma aldosterone concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
Both systolic BP (SBP) and diastolic BP (DBP) measurements were comparable between the different BMI categories (Table 1). SBP negatively correlated with Log‐PRA (r=−.3, P=.009) and positively correlated with Log‐ARR (r=.3, P=.01) but not with Log‐PAC. However, both SBP (r=.3, P=.02, n=42) and DBP (r=.3, P=.03, n=42) correlated positively with Log‐sPAC. Among patients with elevated BP (>140/90 mm Hg), BMI showed a significant quadratic correlation with PAC (r=.5, P=.03; P for r change .04).
3.2. Association between BMI and test results
3.2.1. Full study population
BMI correlated negatively with Log‐PAC and BMI <40 kg/m2 (n=50, r=−.4; P=.02), with a borderline positive correlation with BMI ≥40 kg/m2 (n=9, r=.6; P=.065). BMI showed a significant U‐shaped correlation in the full cohort (r=.45, P=.02; Figure 2A), increasing the r by .12 (P=.02 for change) compared with the linear model. Moreover, BMI showed a significant quadratic correlation with suppressed PAC and a similar trend with suppressed UA levels (Figure 3A and 3B, respectively), with r increasing by .25 (P for r change .05) and .11 (P for r change .07) compared with the linear models, respectively. The analysis results did not change after controlling for plasma potassium levels and when including only women. The quadratic correlation between BMI to random PAC (P=.004) and to PAC after SST (P=.004) remained statistically significant after adjusting for age. No difference was found in BP between obese and nonobese patients (P=.5 for SBP and P=.2 for DBP).
Figure 2.
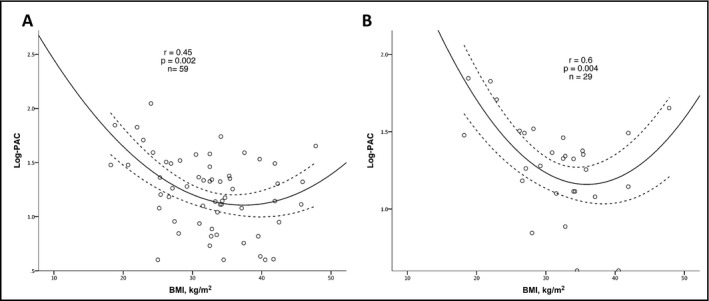
Correlation analysis between body mass index (BMI) and plasma aldosterone concentration (PAC; Log10) in the study population (A), and in patients with confirmed primary aldosteronism (PA) (B). PA was diagnosed according to PAC >10 ng/dL following administration of 2 L of sodium chloride 0.9% over 4 hours, or according to urinary aldosterone (UA) collection >12 μg/24 h following oral sodium loading in the presence of urinary sodium >200 nmol/24 h
Figure 3.
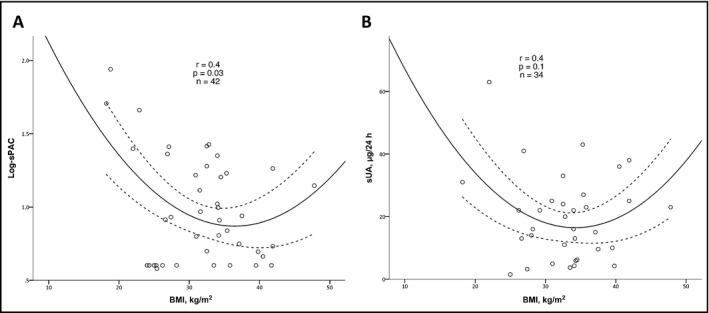
Correlation analysis of body mass index (BMI) with plasma aldosterone concentration (PAC) following saline suppression tests (sPAC) (A) and urinary aldosterone (UA) collection following oral sodium loading (sUA) (B). Primary aldosteronism was diagnosed according to PAC >10 ng/dL following administration of 2 L of sodium chloride 0.9% over 4 hours, or according to UA collection >12 μg/24 h following oral sodium loading in the presence of urinary sodium >200 nmol/24 h
3.2.2. Subgroup analysis according to ARR
Among patients with PA confirmed by SST or oral salt loading, patients with BMI <35 kg/m2 had a trend for higher SBP (>140 mm Hg, 66.7% vs 25%; P=.06), and for higher DBP (>90 mm Hg, 38.1% vs 12.5%; P=.2). Patients with ARR ≥20 (n=28, 47.5%) had higher SBP (142±16 vs 131±18 mm Hg, P=.02) when compared with patients with a lower ARR. Mean DBP was comparable (82±13 vs 77±11 mm Hg, not significant), as was mean BMI (31.1±5.8 vs 33.9±7.4 kg/m2, not significant). The correlation coefficient for BMI and Log‐PAC (r=.6, P=.001) was higher using a quadratic compared with a linear model (P for r change<.001), which was also seen with BMI and Log‐PRA (r=.6, P=.01; P for r change .02). Similarly, sPAC levels had a stronger correlation with BMI using a quadratic model (r=.7, P=.002; P for r change .07). Moreover, among patients with lower ARR <20, the quadratic model showed a better correlation between BMI and both Log‐PAC (r change .4, P for r change <.001) and Log‐PRA (r change .2, P for r change .02).
3.2.3. Subgroup analysis according to confirmatory testing
Twenty‐nine patients (29 of 46, 63.0%) had a confirmed diagnosis of PA either by SST (17 of 42) or oral sodium loading (23 of 34), including 8 patients with positive results on both tests. Among all patients with confirmed PA, BMI had a strong quadratic correlation with Log‐PAC (r=.6, P=.004; Figure 2B), increasing the r for the correlation by .2 compared with the linear correlation analysis (P for change .008). Moreover, BMI had a quadratic correlation with sPAC (r=.6, P=.05), increasing the r coefficient by .1 compared with the linear model (P for change .06). BMI and Log‐PRA did not correlate, probably because of the high rate of undetectable PRA values (23 of 29, 79%).
3.3. Impact of different BMIs on ARR accuracy
The receiver operating characteristic curve for the full study population (n=46, area under the receiver operating characteristic curve=0.798; Figure 4A) and for patients with BMI <30 kg/m2 (n=18) and ≥30 kg/m2 (n=27) revealed excellent accuracy of ARR for case detection of PA among patients with BMI <30 kg/m2 (area under the receiver operating characteristic curve=0.970, Figure 4B), compared with a lower accuracy among patients with obesity (area under the receiver operating characteristic curve=0.621, Figure 4C). The diagnosis of PA was defined as PAC ≥10 ng/dL following SST. However, similar patterns were found using lower PAC cutoff values: ARR accuracy for PA diagnosis in patients with BMI ≥30 kg/m2, <30 kg/m2, or both were 0.959, 1.0, and 0.922 for PAC ≥5 ng/dL, and 0.906, 1.0, and 0.844 for PAC ≥7 ng/dL, respectively.
Figure 4.
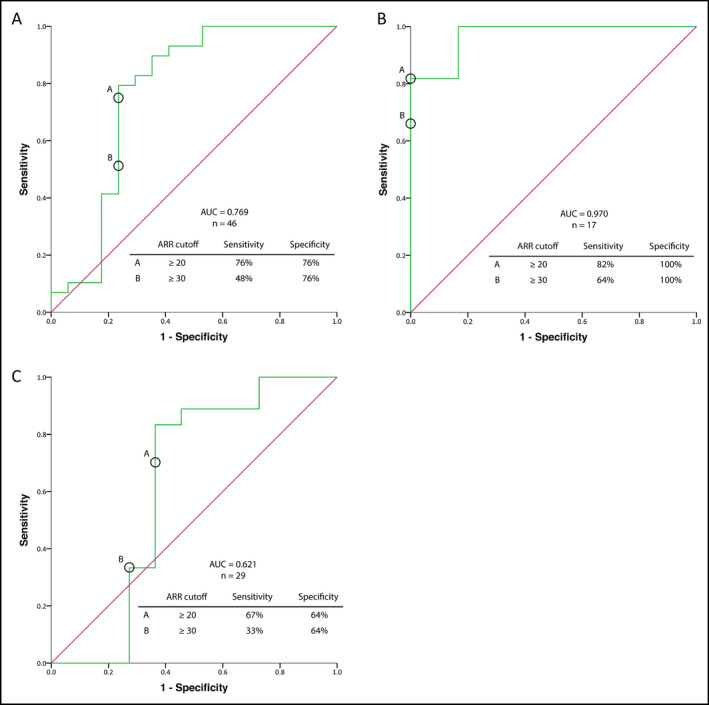
Evaluation of the accuracy of aldosterone to renin ratio (ARR) for the diagnosis of primary aldosteronism (PA) using receiver operating curve analysis in the full study cohort (A), in patients with body mass index (BMI) <30 kg/m2 (B), and in patients with BMI ≥30 kg/m2 (C). PA was diagnosed according to plasma aldosterone concentration >10 ng/dL following administration of 2 L of sodium chloride 0.9% over 4 hours, or according to urinary aldosterone collection >12 μg/24 h following oral sodium loading in the presence of urinary sodium >200 nmol/24 h. AUC indicates area under the receiver operating characteristic curve
The positive predictive value (PPV) for ARR ≥20 and ARR ≥30 among patients with BMI <30 kg/m2 was 100%, whereas the parallel values among patients with obesity were 76.5% and 63.6%, respectively. Similarly, the calculated NPV of low ARR were lower among patients with higher vs lower BMI groups (Table 2).
Table 2.
Positive and negative predictive values of ARR cutoffs for the diagnosis of PAa in the full cohort, with (BMI ≥30 kg/m2) and without (BMI <30 kg/m2) obesity
| ARR cutoff | ≥20 or <20 | ≥30 or <30 | ||
|---|---|---|---|---|
| PPV, % | NPV, % | PPV, % | NPV, % | |
| All patientsb (N=46) | 84.6 | 65.0 | 77.8 | 46.4 |
| BMI ≥30 (n=29) | 76.5 | 58.3 | 63.6 | 38.9 |
| BMI <30 (n=17) | 100 | 75.0 | 100 | 60.0 |
Primary aldosteronism (PA) was diagnosed according to PA concentration >10 ng/dL following administration of 2 L of sodium chloride 0.9% over 4 hours, or according to urinary aldosterone collection >12 μg/24 h following oral sodium loading in the presence of urinary sodium >200 nmol/24 h.
Only patients who underwent case validation tests.
Abbreviations: ARR, aldosterone to renin ratio; BMI, body mass index; NPV, negative predictive value; PPV, positive predictive value.
4. DISCUSSION
Our analysis evaluated for the first time the accuracy of case detection of PA among patients with a wide range of BMI values. We showed complex associations between BMI and PAC, with a negative correlation for BMI <40 kg/m2 and a higher accuracy of quadratic correlation between BMI and PAC, when compared with linear models. This association was also found among patients with elevated (≥20) and nonelevated ARR, hypertension (>140/90 mm Hg), and confirmed PA. Importantly, high ARR yielded lower PPV and NPV for PA diagnosis among patients with obesity (BMI ≥30 kg/m2), with lower accuracy, when compared with the lower BMI cohort. Clinically, this translates to excellent PPV (100%) for ARR among patients with low BMI and lower NPV of ARR among patients with obesity, which may weaken the utility of ARR for case detection of PA in patients with a BMI ≥30 kg/m2 when SST or oral salt loading is used for confirmation. In addition, we showed that the highest accuracy for case detection of PA was achieved using an aldosterone cutoff level of 5 ng/dL or 7 ng/dL, when compared with 10 ng/dL during SST, for all BMI categories.
Studies examining the effects of BMI values on PAC have yielded inconsistent results.6 Goodfriend and colleagues10 reported on the positive correlation between BMI and PAC among women and both sexes. Bentley‐Lewis and coworkers11 described higher UA levels but no difference in PAC among patients with vs patients without obesity. Two studies showed that higher PACs were observed among patients with severe obesity ([BMI >35 kg/m2]12 and [mean BMI 47.8 kg/m2]16), although a study by O'Seaghdha and colleagues17 did not find associations between BMI and PAC, PRA, or ARR in a large cohort. A recent study by Joseph and colleagues13 divided patients into PAC quintiles and showed a higher mean BMI in the lower compared with the second quintiles, in line with our results. A thorough review of the literature6 suggested that a nonlinear relationship between BMI and PAC might explain this inconsistency, which is in keeping with our current analysis and supporting a quadratic rather than linear correlation between BMI and PAC, PRA, or ARR.
Several basic and clinical studies on various populations, including patients with PA, have described the complex relationship between obesity and the renin‐angiotensin system. These observations may in part explain the quadratic association observed between BMI and PRA or PAC. Renin secretion among individuals with obesity is increased by sympathetic nerve activity18 and peroxisome proliferator–activated receptor γ stimulation19 and decreased by estrogen therapy20 through estrogen receptor‐β stimulation.21 Aldosterone can be directly stimulated by adipokines (eg, CTRP‐1), which are secreted from adipose tissues by sensitizing normal adrenal and adenoma cells to angiotensin II,22, 23, 24 suggesting a role for adipose tissue in regulating aldosterone secretion. Adipocytes have direct renin‐like activity and are capable of converting angiotensin to angiotensin I and angiotensin II.25 It is unclear whether angiotensin I and angiotensin II lead to increased aldosterone production in patients with PA, since the process is rather autonomous. Moreover, it is unclear whether excess estrogen levels, which is observed in patients with obesity, might account for a lower PAC through either increased liver metabolism or decreased adrenal secretion (and hence a lower ARR, leading to false‐negative screening). Thus, the mechanism underlying the quadratic association observed between BMI and PRA or PAC in our study requires further research, and the role of estrogens in aldosterone secretion and metabolism should be clarified.
Our analysis showed that BMI is an important factor in the case detection and confirmation of PA. Our findings of higher PPV and NPV among patients with lower BMI (Table 2 and Figure 4) are reassuring. However, accuracy of testing for PA seems inferior in obese patients undergoing evaluation for PA. The comparison of ARR accuracy and predictability between different studies is limited as a result of differences in inclusion criteria, sampling conditions (supine or upright), and ARR cutoff values. This is reflected by the wide range of PPV (34%–75%),26, 27 NPV (84.8%–88.0%),26, 27 and area under the receiver operating characteristic curve (0.80–0.97)26, 28, 29, 30 reported to date. Only a few studies have performed confirmatory testing for negatively screened patients.31 Although the diagnosis of PA in obese patients could be problematic, there are no alternative biochemical strategies for case detection at present. However, although the commonly employed ARR is ≥30,1 our study suggests that with obesity, a threshold of ≥20 could be used for a higher PPV yield (76.5% vs 63.6%). Our data also suggest that among patients with BMI <30 kg/m2, a high ARR possibly obviates the need for confirmatory testing, whereas among obese patients, ARR is less accurate. Thus, we recommend a high index of suspicion for PA in this population. In order to have more accurate practical recommendations for the confirmation of PA, future studies with larger sample sizes and using a variety of confirmatory tests are required. In addition, ascertainment of normal reference values for PAC and PRA per BMI category in normal individuals, patients with hypertension, and patients with PA is required, which might increase the reliability of ARR in the case detection of PA, particularly in patients with obesity.
5. STUDY LIMITATIONS
Despite the strengths of the current analysis in its research plan and data analysis, several drawbacks exist. First, the sample size was small and may not be generalizable to all forms of PA and a selection bias could not be ruled out given the nature of our referral center. Second, both the lowest and highest BMI strata consisted mostly of women, although the results showed similar correlation patterns. Third, there are limitations of a prespecified ARR of 20 or 30 for case detection of PA. Moreover, the results of the SST and oral sodium suppression examinations as confirmatory tests for PA are not definitive for the diagnosis of PA and have their limitations.32 However, our study was designed and approved for these confirmatory tests only. Thus, validating our results using the various confirmatory tests as surrogates for the diagnosis of PA is required. Fourth, measurements of angiotensin I, angiotensin II, estrogens, and variables for calculation of insulin resistance were not available for comparison between the groups, which would have enabled further analysis. Fifth, within‐subject variability in PAC measures, which is not uncommon in PA, could have affected the single‐measure correlation validity of our analysis. Finally, although we did not find a relationship between BP and BMI, this might be explained by the fact that the patients were medically treated before being included in the study.
6. CONCLUSIONS
Our study shows that BMI has a nonlinear correlation with PAC, PRA, and ARR, as well as with confirmatory tests results. This pattern was found among patients with a positive case detection test (ARR ≥20) and confirmed PA. The PPV, NPV, and test accuracy of ARR were higher among patients with BMI <30 kg/m2 when compared with obese patients, which may affect the diagnosis of PA in this BMI group. Further studies are required to confirm this important association.
CONFLICT OF INTEREST
The authors have nothing to disclose.
Tirosh A, Hannah‐Shmouni F, Lyssikatos C, et al. Obesity and the diagnostic accuracy for primary aldosteronism. J Clin Hypertens. 2017;19:790‐797. 10.1111/jch.13041
Funding information
This research was supported in part by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
REFERENCES
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889‐1916. [DOI] [PubMed] [Google Scholar]
- 2. Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293‐2300. [DOI] [PubMed] [Google Scholar]
- 3. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243‐1248. [DOI] [PubMed] [Google Scholar]
- 4. Funder JW. Primary aldosteronism as a public health issue. Lancet Diabetes Endocrinol. 2016;4:972‐973. [DOI] [PubMed] [Google Scholar]
- 5. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity‐associated hypertension. J Hum Hypertens. Nature Publishing Group. 2014;28:3‐9. [DOI] [PubMed] [Google Scholar]
- 7. Fallo F, Pilon C, Urbanet R. Primary aldosteronism and metabolic syndrome. Horm Metab Res. 2012;44:208‐214. [DOI] [PubMed] [Google Scholar]
- 8. Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355‐362. [DOI] [PubMed] [Google Scholar]
- 9. Lamounier‐Zepter V, Rotthoff T, Ansurudeen I, et al. Increased aldosterone/renin quotient in obese hypertensive women: a novel role for low‐density lipoproteins? Horm Metab Res. 2006;38:471‐475. [DOI] [PubMed] [Google Scholar]
- 10. Goodfriend TL, Egan BM, Kelley DE. Aldosterone in obesity. Endocr Res. 1998;24:789‐796. [DOI] [PubMed] [Google Scholar]
- 11. Bentley‐Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high‐salt diet. J Clin Endocrinol Metab. 2007;92:4472‐4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarzani R, Guerra F, Mancinelli L, Buglioni A, Franchi E, Dessì‐Fulgheri P. Plasma aldosterone is increased in class 2 and 3 obese essential hypertensive patients despite drug treatment. Am J Hypertens. 2012;25:818‐826. [DOI] [PubMed] [Google Scholar]
- 13. Joseph JJ, Echouffo‐Tcheugui JB, Kalyani RR, et al. Aldosterone, renin, and diabetes mellitus in African Americans: the Jackson Heart Study. J Clin Endocrinol Metab. 2016;101:1770‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zilbermint M, Xekouki P, Faucz FR, et al. Primary aldosteronism and ARMC5 variants. J Clin Endocrinol Metab. 2015;100:E900‐E909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 16. Andronico G, Cottone S, Mangano MT, et al. Insulin, renin‐aldosterone system and blood pressure in obese people. Int J Obes Relat Metab Disord. 2001;25:239‐242. [DOI] [PubMed] [Google Scholar]
- 17. O'Seaghdha CM, Hwang SJ, Vasan RS, et al. Correlation of renin angiotensin and aldosterone system activity with subcutaneous and visceral adiposity: the Framingham Heart Study. BMC Endocr Disord. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533‐2536. [DOI] [PubMed] [Google Scholar]
- 19. Todorov VT. PPARgamma‐dependent control of renin expression: molecular mechanisms and pathophysiological relevance. PPAR Res. 2013;2013:451016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin‐angiotensin system in postmenopausal women. Circulation. 1997;95:39‐45. [DOI] [PubMed] [Google Scholar]
- 21. Caroccia B, Seccia TM, Campos AG, et al. GPER‐1 and estrogen receptor‐β ligands modulate aldosterone synthesis. Endocrinology. 2014;155:4296‐4304. [DOI] [PubMed] [Google Scholar]
- 22. Ehrhart‐Bornstein M, Lamounier‐Zepter V, Schraven A, et al. Human adipocytes secrete mineralocorticoid‐releasing factors. Proc Natl Acad Sci USA. 2003;100:14211‐14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeon JH, Kim K, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008;22:1502‐1511. [DOI] [PubMed] [Google Scholar]
- 24. Rossi GP, Sticchi D, Giuliani L, et al. Adiponectin receptor expression in the human adrenal cortex and aldosterone‐producing adenomas. Int J Mol Med. 2006;17:975‐980. [PubMed] [Google Scholar]
- 25. Saye JA, Ragsdale NV, Carey RM, Peach MJ. Localization of angiotensin peptide‐forming enzymes of 3T3‐F442A adipocytes. Am J Physiol. 1993;264:C1570‐C1576. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz GL, Chapman AB, Boerwinkle E, Kisabeth RM, Turner ST. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clin Chem. 2002;48:1919‐1923. [PubMed] [Google Scholar]
- 27. Jansen PM, van den Born BJ, Frenkel WJ, et al. Test characteristics of the aldosterone‐to‐renin ratio as a screening test for primary aldosteronism. J Hypertens. 2014;32:115‐126. [DOI] [PubMed] [Google Scholar]
- 28. Tiu SC, Choi CH, Shek CC, et al. The use of aldosterone‐renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90:72‐78. [DOI] [PubMed] [Google Scholar]
- 29. Ducher M, Mounier‐Véhier C, Baguet JP, et al. Aldosterone‐to‐renin ratio for diagnosing aldosterone‐producing adenoma: a multicentre study. Arch Cardiovasc Dis. 2012;105:623‐630. [DOI] [PubMed] [Google Scholar]
- 30. Kuo CC, Wu VC, Tsai CW, et al. Combining body mass index and serum potassium to urine potassium clearance ratio is an alternative method to predict primary aldosteronism. Clin Chim Acta. 2011;412:1637‐1642. [DOI] [PubMed] [Google Scholar]
- 31. Montori VM, Young WF. Use of plasma aldosterone concentration‐to‐plasma renin activity ratio as a screening test for primary aldosteronism. A systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31:619‐632, xi. [DOI] [PubMed] [Google Scholar]
- 32. Rossi GP, Belfiore A, Bernini G, et al. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone‐producing adenoma. J Hypertens. 2007;25:1433‐1442. [DOI] [PubMed] [Google Scholar]


