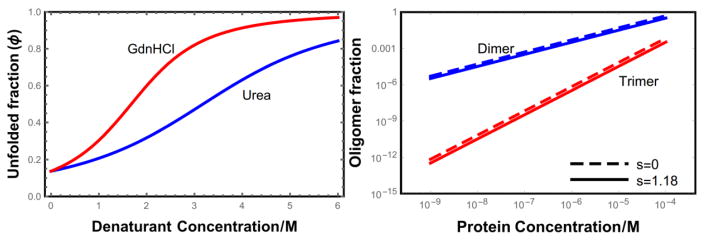
Figure 2.
(a) Denaturant unfolding curves for isolated helices. For the parameters given in the text, helices are ~86% folded in the absence of denaturant. We expect that real proteins will show a more cooperative transition than the helix coil model meaning that they are more folded in the absence of denaturant and the unfolding transition will be more abrupt[29]. (b) Fraction of L = 100 helical proteins in the dimer state (2c2/ct) and in the trimer state (3c3/ct) as a function of the total protein concentration.