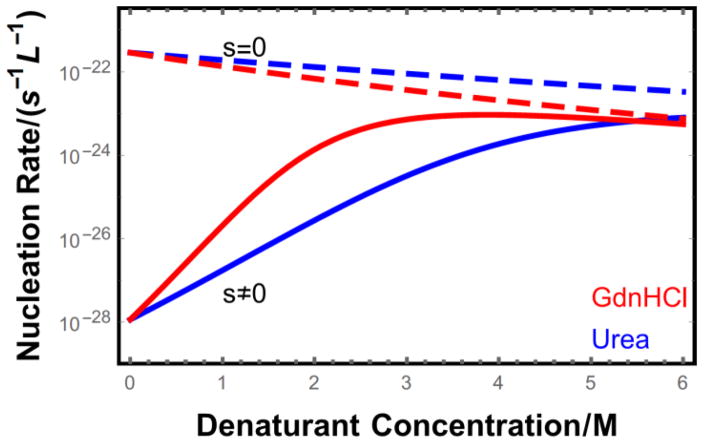
Figure 4.
Nucleation rate as a function of denaturant concentration for proteins of length L = 100 at a concentration ct = 10−5 M. Intrinsically disordered proteins (s = 0) are monotonically inhibited from nucleating by the addition of denaturant due to the weakening of intermolecular bonds. In contrast, proteins with stable folded states (solid lines) show greatly enhanced nucleation upon denaturant addition because of the increased population of unfolded proteins (s = 1.16 at cd = 0). This trend reverses at high denaturant concentrations since the proteins are mostly unfolded and the dominant effect of further denaturant addition is the destabilization of fibril contacts.