Figure 4.
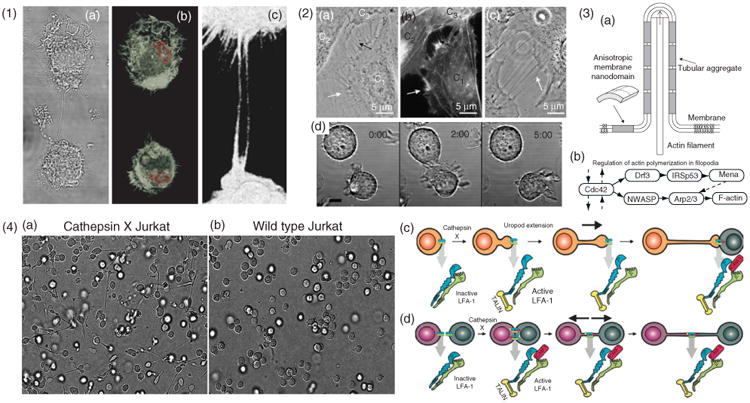
Cytoskeletal structure and formation of ICNs (1) Actin structure of ICNs illustrated with macrophages, (a), differential interference contrast micrograph, (b), micrograph showing a 3D rendered confocal stack. F-actin stained by phalloidin (white) and the nucleus stained with Hoescht (red). (c), A zoomed-in region showing the phalloidin stained nanotubes. (2) Formation of ICNs. (a–c), Bridging nanotubes. (a), Phase contrast image of live T24 cells, (b), fluorescence micrograph with actin labeling of the same cells as in A. The white arrows in (a) and (b) indicate short and dynamic membrane protrusions with which the approaching cell explores its surroundings. The black arrow in, A, points at protrusions that have already connected to the target cell. Bridging nanotubes can be more than 20 μm in length and bifurcations are occasionally observed (arrow in C). (Reprinted with permission from Ref 51. Copyright 2008 Elsevier). (d), Time-lapse imaging of 721.221 cells forming a transient contact demonstrates that a connecting nanotube can form as cells move apart after contact. Scale bar, 10 μm. (Reprinted with permission from Ref 5. Copyright 2004 AAI). (3) Schematic illustration, (a), of stabilization of nanotubular membrane protrusions by accumulation of anisotropic membrane nanodomains in the tubular region. The growing actin filaments push the membrane outward. The protrusion could additionally be stabilized by accumulated anisotropic nanodomains that favor an anisotropic cylindrical geometry of the membrane. The cylindrical-shaped anisotropic membrane domains, once assembled in the membrane region of a nanotubular membrane protrusion, keep the protrusion mechanically stable even if the cytoskeletal components (actin filaments) are disintegrated. (Reprinted with permission from Ref 51. Copyright 2008 Elsevier). (b), The general regulatory pathway of actin polymerization in filopodia46 (4) LFA-1 activation is responsible for two distinct pathways of nanotube formation in Jurkat T-cells. (a), Example of the extension and nanotube outgrowth by Cathepsin X-up regulated Jurkat T-cells in a three-dimensional environment, leading to increased ICN-mediated cell-to-cell contact. (b), Wt cells remained in a spherical shape. Nanotubes form upon uropod elongation in the absence of prior intercellular contact. (c), The proposed mechanism of nanotube outgrowth via persistent LFA-1 activation (mediated by cathepsin X). Talin binds to the cytoplasmic tail of LFA-1, followed by binding of ICAM-1 to the extracellular domain, enabling inside-out driven actin reorganization, uropod formation, elongation, and ICN formation. (d), The proposed mechanism of nanotube formation following intercellular contact between T-cells. LFA-1/ICAM-1 interactions arising in T-cell aggregation are enhanced in cathepsin X-upregulated Jurkat cells. Prolonged LFA-1 activation enables cytoskeletal reorganization, similar to that associated with uropod outgrowth, with talin-binding to the cytoplasmic tail of LFA-1 and subsequent membrane nanotube formation as cells depart. (Modified with permission from Ref 52. Copyright 2009 Springer Science+Business Media).
