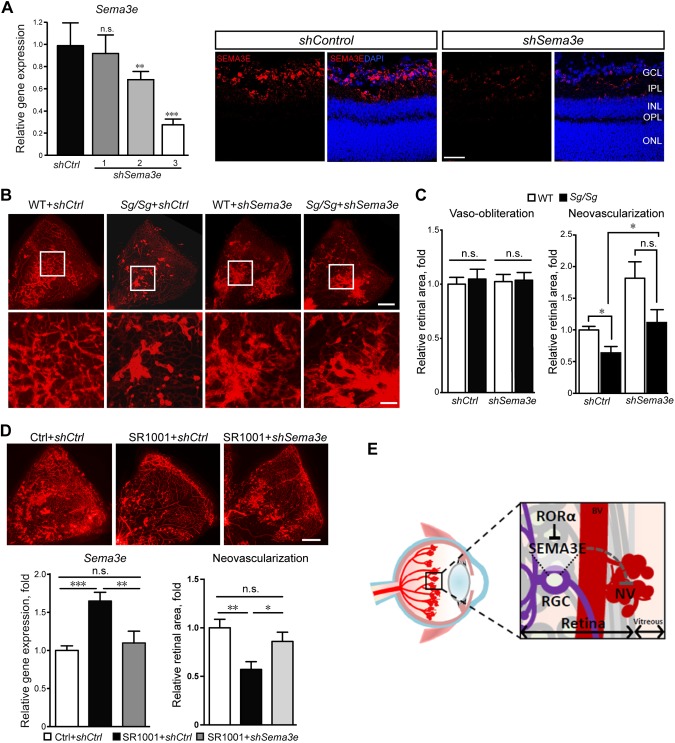
Figure 5.
RORα-Sema3e axis regulated neurovascular interaction in pathological retinal angiogenesis. A) AAV2-shSema3e (shSema3e) effectively knocked down retinal Sema3e expression compared with AAV2-sh control (shCtrl) at P16. The knockdown efficiency was validated by real-time qPCR analysis (left) and immunofluorescence analysis (right). AAV2-shSema3e-3 was selected for experiments in panel B (n = 6/group). B) Representative P17 retinal whole-mounts from Sg/Sg and WT mice with OIR treated with AAV2-shSema3e and AAV2-shCtrl in contralateral eyes, stained with isolectin IB4 (red) for blood vessels. Selected retinal areas (white box) were enlarged to show pathological neovessels (bottom panel). Scale bars, 500 μm (top) and 125 μm (bottom). C) The levels of vaso-obliteration (left panel) were comparable between AAV2-shSema3e and AAV2-shCtrl treated P17 OIR retinas in both Sg/Sg and WT groups. Quantified levels of pathological neovascularization (right panel) were significantly decreased in Sg/Sg OIR retinas compared with WT, both with AAV2-shCtrl treatment. AAV2-shSema3e treatment largely abolished the decrease of pathological neovascularization in Sg/Sg OIR retinas compared with AAV2-shCtrl–treated Sg/Sg retinas. Levels of neovascularization were expressed as percentage of total retinal areas (n = 8 in WT group; n = 6 in Sg/Sg group). D) SR1001 treatment induced retinal expression of Sema3e (n = 5/group), which was suppressed by AAV-shSema3e treatment. AAV-shSema3e treatment significantly increased the levels of pathological neovascularization in SR1001-treated OIR mice compared with AAV-shCtrl (n = 11/group). E) Schematic illustration of the role of neuronal RORα in pathological ocular neovascularization. In normal retinas, RORα levels are low and SEMA3E expression levels are high, which suppresses blood vessel growth and maintains quiescence of normal retinal vessels. In retinopathy, RORα induction in RGC represses transcription of SEMA3E, leading to decreased SEMA3E levels and relieving its inhibition of pathological vessel growth, thereby resulting in increased pathological neovascularization. Targeted suppression of RORα may promote SEMA3E signaling–mediated neurovascular interaction and protect pathological neovascularization in retinopathy. Data are presented as means ± sem. BV, blood vessel; n.s., no significance; NV, neovessel; RGC, retinal ganglion cell. *P < 0.05, **P < 0.01, ***P < 0.001.