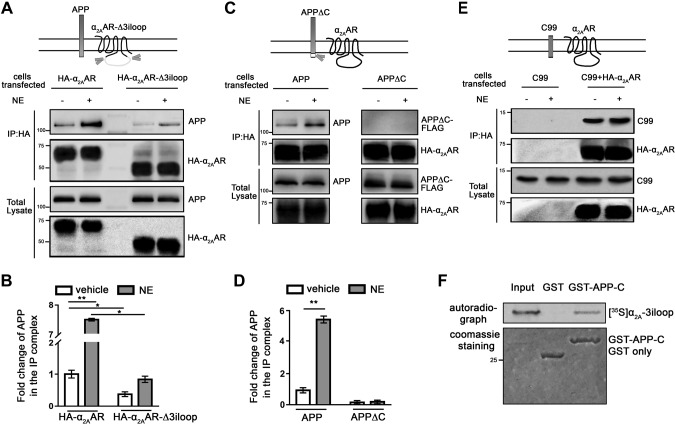
Figure 3.
APP and α2AAR interact through their intracellular domains. A, B) Deletion of the α2AAR 3iloop significantly reduced its ability to interact with APP. HEK293 cells coexpressing APP with HA-α2AAR or HA-α2AAR-Δ3iloop were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) or vehicle for 5 min. Cell lysates were subjected to IP assays with an HA Ab. A) Representative Western blots. B) Quantification of the amount of APP in the IP complex. Data (means ± sem) are expressed as fold change vs. vehicle control (defined as 1.0) (n = 3 in each group). *P < 0.05; **P < 0.01 (Student’s t test). C, D) Removal of the APP-C abolished its interaction with the α2AAR. HEK293 cells coexpressing HA-α2AAR with APP or APPΔC were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) or vehicle for 5 min. Cell lysates were subjected to IP assays. C) Representative Western blots. D) Quantification of the amount of APP and APPΔC in the IP complex. Data (means ± sem) are expressed as fold change vs. vehicle control (defined as 1.0) (n = 3 in each group). **P < 0.01 (Student’s t test). E) The C99 fragment of APP formed a complex with HA-α2AAR. HEK293 cells coexpressing C99, with or without HA-α2AAR were stimulated with vehicle or 10 µM NE (plus 1 µM prazosin and 1 µM propranolol), and cell lysates were subjected to IP assays. Representative Western blots are shown. F) In vitro GST pull-down assay showing direct interaction between the α2AAR 3iloop and APP C-terminal domain. Purified GST or GST-fused APP-C was incubated with [35S]-labeled, in vitro translated α2AAR-3iloop. Input probe represents one-tenth of the total input in each reaction.