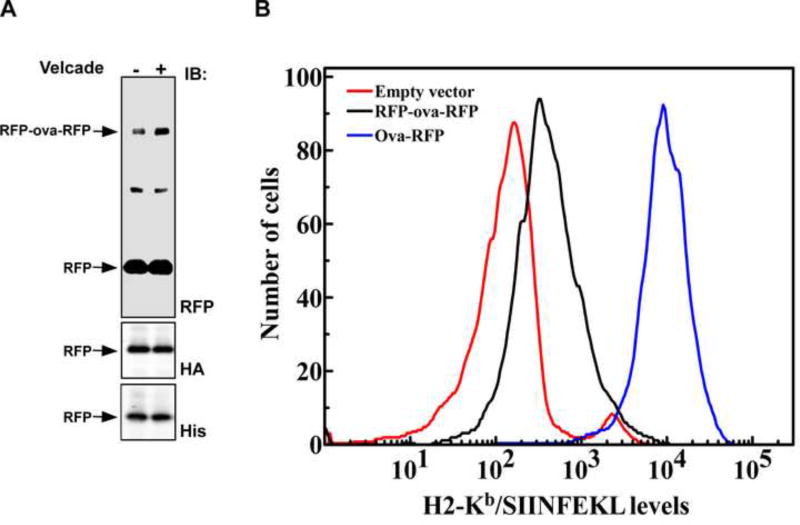
Figure 6. SIINFEKL is presented on surface H2-Kb molecules of EL4 cells expressing ova-RFP but much less in cells expressing RFP-ova-RFP.
(A) Western blot analysis of total cell lysate prepared from EL4 cells expressing HA-RFP-ova-RFP-His in the presence and absence of Velcade. The much greater content (more than a hundred fold) of the intact RFP domains compared to the full-length substrates demonstrates that RFP is quantitatively released and not degraded during the in vivo proteasomal degradation of RFP-ova-RFP. Both the N-terminal and the C-terminal RFP domains were spared, as is evident from the immunoreactivity of the RFP domains with HA and His antibodies. (B) EL4 cells expressing ova-RFP (blue), RFP-ova-RFP (black) or empty vector (red), were stained with 25D1.16 monoclonal antibody, recognizing the H2-Kb/SIINFEKL complex. For detection, Alexa Fluor 647-conjugated anti-mouse was used as a secondary antibody. Live cells were gated based on their light scatter characteristics. To ensure similar levels of expression, a narrow GFP (pMIG vector internal iris) gate was selected.