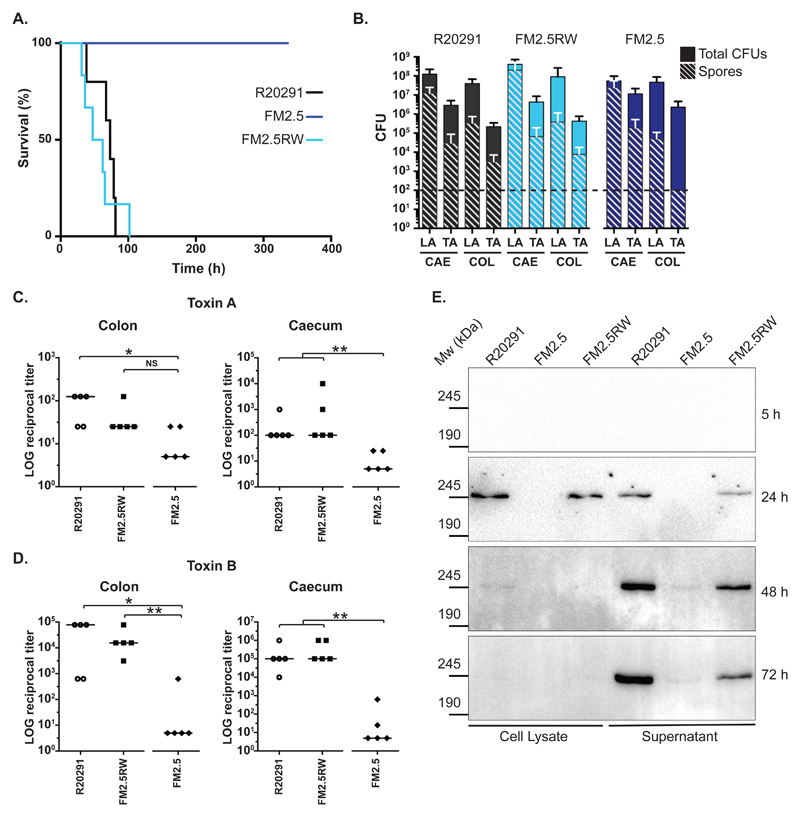
Fig. 4.
In vivo analysis of slpA mutant in the Syrian Golden hamster. (A) Times to experimental endpoint of animals infected with R20291 (black line), FM2.5 (dark blue line) and FM2.5RW (light blue line) respectively. Each line represents 6 animals. (B) Total CFUs and spore CFUs (following heat treatment at 56°C for 20 min) were determined for Lumen- (LA) and tissue-associated (TA) bacteria recovered from caecum (CAE) and colon (COL) of infected animals and quantified at experimental endpoint (R20291 and FM2.5RW) or at 14 days post-infection (FM2.5). Shown are the mean and standard error. The horizontal dotted line indicates the limit of detection. None of the observed differences, including those for the TA-spore CFUs from the colon, are statistically significant. (C and D) Relative toxin activity of filtered gut samples on HT-29 (toxin A) and Vero cells (toxin B) respectively. Values represent the reciprocal of the first dilution in which cell morphology was indistinguishable from untreated wells. Samples were taken at experimental endpoint (R20291 and FM2.5RW) or at 14 days post-infection (FM2.5). (* = P <0.05, ** = P <0.01, NS = not significant, determined using using a two-tailed nonparametric Mann-Whitney test. (E) In vitro cell lysate and culture supernatant samples from R20291, FM2.5 and FM2.5RW were normalized to an equivalent optical density and separated on 6% SDS polyacrylamide gels. Toxin B was detected by Western immunoblot using an anti-Toxin B monoclonal antibody. Samples were taken at the indicated time points.