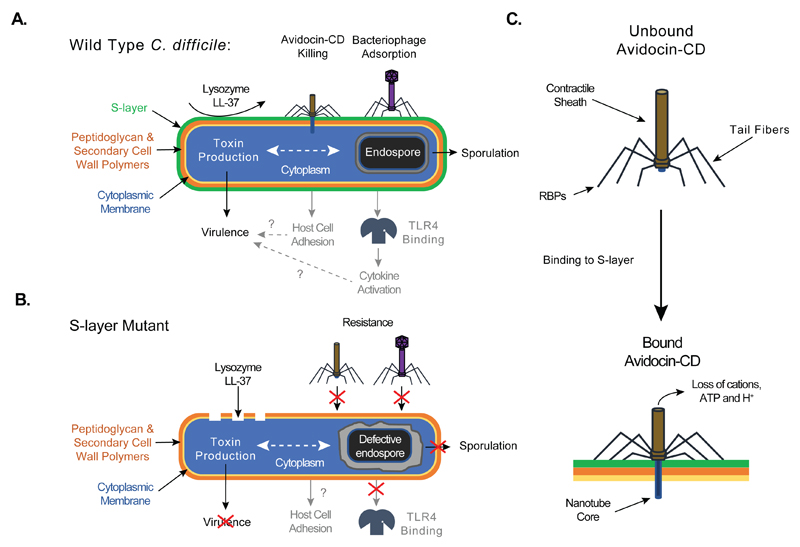
Fig. 5.
Schematic diagram depicting the phenotypes of Wild Type (A) and S-layer null mutant (B) cells in C. difficile biology and pathophysiology. Processes labeled in black indicate new functions discerned in this manuscript. Processes labeled in grey indicate previously identified functions. Dotted lines indicate relationships that need to be studied further. Question marks indicate previously described connections incompatible with observations made with the S-layer null mutant and warranting further investigation. (C) Schematic of Avidocin-CD structure and function. Binding to C. difficile by the Avidocin-CD receptor binding proteins (RBPs) triggers the sheath to contract and force the hollow nanotube core across the cell envelope. The resulting pore allows small metabolites, such as protons, ATP and cations, to escape from the cell cytoplasm, which, in turn, disrupts the cell’s membrane potential and kills the cell.