Abstract
Pathological gambling (PG) is a common and costly public health problem associated with impaired quality of life and high suicide rates. Despite its frequency in the general population, PG course is poorly understood in older adults who are especially vulnerable to its devastating consequences. We enrolled 175 subjects in a longitudinal study of gambling behavior: our case group of 53 older adults with PG (≥ 60 years), and two comparison groups including 72 younger adults with PG (< 40 years) and 50 older adults without PG (≥ 60 years). Subjects with PG met lifetime criteria for DSM-IV PG and had a South Oaks Gambling Screen (SOGS) and National Opinion Research Center DSM Screen for Gambling Problems (NODS) scores ≥5. Subjects were evaluated at intake and reassessed every 6 months and drop outs were replaced. Follow-up lasted a mean (SD) of 2.6 (1.4) years. At intake older PGs were more likely to be female, Caucasian, divorced, and to have a lower level of education. Older and younger PGs were similar in gambling severity, but older PGs were more likely to have sought PG treatment. Older PGs had lower rates of lifetime drug use disorders, attention deficit/hyperactivity disorder, and obsessive-compulsive disorder. They preferred slots, were more likely to receive PG treatment, and were less likely to discontinue participation in the study. Week by week gambling activity levels showed a significant general downward movement for older and younger PGs, although there were no differences between the groups. Elders without PG had no change in their level of gambling activity. We conclude that younger and older PGs moved toward a reduced level of gambling activity during follow-up. Our data challenge the notion that PG is chronic and progressive.
Keywords: pathological gambling, prospective follow-up studies, older adults, elders
1. Introduction
Pathological gambling (PG) is characterized by the presence of persistent and recurrent maladaptive gambling behavior the person is unable to adequately control (American Psychiatric Association, 1994). First recognized in DSM-III (American Psychiatric Association, 1980), PG was classified as a disorder of impulse control. In DSM-5 (American Psychiatric Association, 2013), the disorder was moved to the chapter on substance-related and addictive disorders. The reclassification recognized the growing recognition of clinical, genetic and neurobiological overlap between traditional alcohol and drug use disorders and PG (Black and Grant, 2014). The name also was changed to gambling disorder to reduce the stigma attached to the word “pathological.” The term pathological gambling (and the term pathological gamblers, or PGs) is used herein, rather than gambling disorder, because our research was based on the DSM-IV definition (American Psychiatric Association, 1994).
PG prevalence is estimated at 1% – 2% of the general population, but the prevalence of subclinical PG (“at-risk” gambling) is higher (Kessler et al., 2007). Surprisingly, prevalence rates are still higher in adolescent (3%–8%) and college populations 4%–14%) (NORC, 1999; Welte et al., 2001; Clark, 2012). PG has a mean onset at 34 years, but can occur at any age including senescence (Black et al., 2015). Prevalence rates are higher in men than women in whom the disorder starts later and progresses more rapidly (Taveres et al., 2003). PG is usually accompanied by the presence of co-occurring disorders, including substance use disorders, mood and anxiety disorders, and personality disorders (Kessler et al., 2008; Lorains et al; 2011; Black et al., 2014).
PG is especially problematic in older adults (or “elders”) because its rate in this subgroup has increased more rapidly than in any other segment of the US population (NORC, 1999). The lifestyle of many elders combined with the availability of time and financial resources (e.g., Social Security income, pensions) contributes to their vulnerability (Goskar, 1999; McNeilly and Burke, 2001; Grant et al., 2001; Pavalko, 2002). Casinos have been known to entice seniors with transportation, free drinks, free rooms, meal tokens, and medication discounts (Goskar, 1999). Older adult gamblers are also at greater risk for physical/emotional problems than their non-problem gambling peers, as well as atherosclerotic disease and heart conditions (Erickson et al., 2005; Pilver and Potenza, 2013).
Despite its public health significance, little is known about the course of PG in older adults. Questions remain including whether PG in elders fluctuates in severity and intensity and if gambling behavior spontaneously remits. While DSM-5 promotes the view that PG is chronic and deteriorating, these views have been challenged (Slutske, 2006; Sartor et al., 2007; LaPlante et al., 2008; Edgerton et al., 2015). Research suggests that PG has a natural ebb and flow with many PGs moving toward reduced gambling involvement or experiencing spontaneous (Slutske et al., 2003; Slutske, 2006; LaPlante et al., 2008). Because none of these reports stratify by age, it is unclear if the course of older adults with PG differs from course experienced in younger adults.
We recently conducted a follow-up study of individuals with PG (Black et al., 2015). While the main focus was gambling behavior in older adults, data were collected on a younger PG comparison group and a group of older adults without PG. Important differences have been identified between younger and older-onset persons with PG. Earlier-onset PGs are more often male, have greater psychiatric comorbidity, and are more interested in strategic (or “action”) games such as card games and sports betting (Grant et al., 2009; Black et al., 2015).
We hypothesized that PG would become less symptomatic or remit during follow-up. We based this hypothesis on our own experience, and the literature. For example, Sartor et al. (2007) assessed gambling symptoms retrospectively in participants in the Vietnam Era Twin Registry study and found that 17% of subjects with PG had periods of abstinence and 43% reported ≥ 5 symptom-free gambling phases. In a review of five follow-up studies, LaPlante et al. (2008) concluded that most persons with PG improved and moved toward a lower gambling level. We also hypothesized that elders with PG would show greater improvement than younger PGs, based in part on work showing that persons with older-onset PG have lower levels of trait impulsiveness (Black et al., 2015).
2. Methods
2.1 Participants and Procedure
We recruited PGs who had participated in our family study (Black et al., 2014), but also recruited subjects through our PG registry, university-wide emails, physician referral, word-of-mouth, and Gamblers Anonymous (GA) meetings. All had South Oaks Gambling Scores (SOGS; Lesieur and Blume, 1987) and National Opinion Research Center DSM Screen for Gambling Problems (NODS; NORC, 1999) scores ≥ 5. All met lifetime DSM-IV PG criteria (American Psychiatric Assocation, 1994). PGs were ≥ 18 and < 40 years or were ≥ 60 years. Subjects had to speak English and could not have a psychotic, cognitive, or chronic neurological disorder. Persons taking dopamine agonists were excluded because the drugs have been linked to the onset/worsening of PG (Lader, 2008). Subjects were classified using the Shaffer and Hall (1996) multi-level scheme (level 0, no gambling; level 1, recreational gambling; level 2, “at-risk” gambling; level 3, PG). Subjects could not have been adopted because family history information would be unavailable. All were asked to provide contact information for two relatives/friends likely to know his/her whereabouts to facilitate follow-up.
Older adults without PG were recruited with the assistance of the Center for Social and Behavioral Research at the University of Northern Iowa (Cedar Falls, IA) using random digit dialing. They were required to be ≥ 60 years and to have a SOGS score of ≤ 2 and a NODS score of 0. The criteria used for their selection were otherwise identical to those used for subjects with PG.
Written informed consent was obtained from all subjects according to procedures approved by the University of Iowa Institutional Review Board. Intake interviews were in-person with follow-up interviews occurring via telephone. We replaced subjects who dropped out (or were lost to follow-up) in their first 24 months of follow-up. This procedure helped to ensure that sample size and statistical power was not compromised because attrition has been acknowledged as a significant problem in PG research (Black et al., 2007; Wohl and Sztainert, 2011). Enrollment began in March 2011 and follow-up visits began approximately 6 months after a subject’s intake interview and continued at 6 month intervals. The last follow-up visits occurred December 31, 2015.
2.2 Study Assessments
Diagnostic instruments included the Structured Clinical Interview for DSM-IV (SCID; Spitzer et al., 1994) and the Family History Research Diagnostic Criteria, adapted to include criteria for PG (Andreasen et al., 1977). The Minnesota Impulsive Disorders Interview (MIDI; Christenson et al., 1994) was used to collect data on impulse control disorders (kleptomania, pyromania, intermittent explosive disorder, and trichotillomania) and several non-DSM conditions (compulsive buying, compulsive sexual behavior, and Internet addiction). Attention deficit/hyperactivity disorder (ADHD) was assessed using a module from the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). The Structured Interview for DSM-IV Personality (SIPD-IV; Pfohl et al., 1997) was used to assess the presence of personality disorders. The Beck Depression Inventory (BDI; Beck et al., 1996) was used to assess depressive symptoms. The Barratt Impulsiveness Scale-Version 11a (BIS; Barratt, 1959) was used to assess motor, cognitive, and non-planning impulsiveness. The Medical Outcome Study Short Form-36 (MOS; Ware et al., 1993) was used to measure physical and mental dimensions of health. The Gambling Symptom Assessment Scale (GSAS; Kim et al., 2001) was used to assess gambling severity/symptoms. We modified the Time Line Follow Back (TLFB; Sobell and Sobell, 1995) to track time spent, amount wagered, and number of gambling episodes.
The Longitudinal Interval Follow-up Evaluation (LIFE; Warshaw et al., 1994) was modified to assess the weekly course of PG, co-occurring disorders, and addictions. Severity ratings were made for each week of the follow-up for each disorder. PG was rated using the gambling levels of Shaffer and Hall: 1) level 0: no gambling; 2) level 1: gambling present, but not problematic; 3) level 2: gambling present; gambling problems are sub-clinical (“at-risk”); and 4) level 3: gambling present, and achieves PG threshold (Shaffer and Hall, 1996). Psychotropic medication usage and psychotherapy participation was recorded, as was clinic visits and hospitalizations. Attendance at Gamblers Anonymous meetings was also recorded. Psychosocial functioning was rated for each month of follow-up. The Global Assessment Scale (GAS; Endicott et al., 1976) is embedded in the LIFE and rated for each month, assessing global functioning (0–100 scale). The following assessments were repeated at each 6-month follow-up visit: the NODS, the GSAS, the MOS, the LES, and the TLFB.
Remission was defined as no gambling for 8-weeks; partial remission was defined as no problematic gambling for 8-weeks; chronic problematic gambling (i.e., continuous, without letup) as problematic gambling or PG for the length of the follow-up. Relapse was defined as ≥ 1 week of problematic gambling or PG following an 8-week remission. Group differences in change in gambling activity level were also examined, where change was defined as any change (upward or downward) in weekly gambling activity classification, relative to the previous week. This allowed the examination of weekly variation in gambling activity levels for older and younger PGs.
2.3 Statistical Analysis
Younger PGs, older PGs, and elders without PG were compared on sociodemographic and clinical characteristics collected at intake. For categorical variables (gender, race/ethnicity, being divorced, educational attainment, gambling preference, mental health treatment and hospitalization, gambling treatment and hospitalization, childhood maltreatment, psychiatric comorbidity, and history of attempted suicide), Pearson’s Chi-Square test (or Fisher’s Exact test, when appropriate) was used to test for differences by group. Comparisons of interest included older PGs vs. younger and older PGs vs. elders without PG. For dimensional variables (e.g., age, age at PG onset), the Mann-Whitney U test was used.
The Cox Proportional Hazards model was used to test for group differences in time to study discontinuation. For each group, PG levels were summarized by the percentage of individuals at each gambling level for each week of the follow-up. Generalized estimating equations (GEE) models were used to compare younger to older PGs over the follow-up on PG course outcomes. Remission, partial remission, and chronic PG outcomes were defined for individuals with 8 or more weeks of follow-up. GEE models assumed the logit link function and first-order autoregressive correlation structure. The Cox Proportional Hazards model is used to test for group differences in time to relapse.
Using the same PG outcomes, younger and older PGs were compared on slopes over time. GEE models were used with effects for time (week of follow-up), group (younger vs. older PGs), and group by time interaction. This analysis tests whether a positive or negative trend in PG course exists in each group, and compares the two groups on trend. Statistical tests were 2-tailed with α = 0.05.
3. Results
The analysis included 175 subjects including older PGs (n = 53), younger PGs (n = 72), and elders without PG (n = 50). Follow-up lasted a mean (SD) of 2.6 (1.4) years but ranged from 0 to 4.6 years. Among the 175 study participants, 53 (30%) discontinued participation. Discontinuation rates were highest for younger PGs (44%), followed by older PGs (25%), and then elders without PG (16%). Nine individuals (4 older PGs, 4 younger PGs, 1 control) chose to withdraw from the study for personal reasons (i.e., did not want further contact, found the interviews inconvenient/time consuming, etc.), 2 controls withdrew because of health concerns, 3 individuals (an older PG and 2 controls) died during follow-up, and 1 control was later removed due to violating the study protocol. Reasons for study discontinuation were unknown for 38 individuals lost to follow-up (8 older PGs, 28 younger PGs, 2 controls). Time to study discontinuation varied across the three groups (Cox Proportional Hazards Chi-Square = 15.0, df = 2, p < 0.001). The hazard ratio for younger PGs (relative to elders without PG) was 3.99 (95% CI, 1.82, 8.73). The hazard ratio for older PGs (relative to elders without PG) was 1.71 (95% CI, 0.71, 4.13). The hazard ratio for younger PGs (relative to older PGs) was 2.34 (95% CI, 1.22, 4.47).
Table 1 shows the comparison of the three groups. Older PGs were younger than older adults without PG (mean 66.5 vs. 71.2 years), were more likely to be divorced (57% vs. 24%), and were less likely to have a college degree (45% vs. 70%). Older PGs also had higher ratings of impulsiveness (BIS) and depressive symptoms (BDI) than older adults without PG; they were more likely to have had a prior psychiatric hospitalizations (25% vs. 4%), to have experienced some form of childhood maltreatment (61% vs. 34%), and to have attempted suicide (19% vs. 4%). Lifetime comorbid mental health disorders were more common in older PGs than older adults without PG, including an alcohol use disorder (53% vs. 18%), any anxiety disorder (42% vs. 20%), any mood disorder (58% vs. 34%), any eating disorder (13% vs. 1%), and any impulse control disorder (ICD; 19% vs. 2%).
Table 1.
A comparison of baseline characteristics for younger PGs, older PGs, and elder controls
| Variables | Younger PGs (n=72) | Older PGs (n=53) | Elder Controls (n=50) | Older vs. Younger PGs | Older PGs vs. Controls | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| X2 | P-value | X2 | P-value | ||||
| PG age at onset, years, mean (SD) | 22.1 (7.3) | 50.0 (13.2) | 55.6 | <0.001 | |||
| Age, mean (SD) | 27.4 (7.7) | 66.5 (6.8) | 71.2 (9.2) | 88.2 | <0.001 | 7.0 | 0.008 |
| Female, no. (%) | 14 (19%) | 34 (64%) | 28 (56%) | 25.8 | <0.001 | 0.7 | 0.398 |
| European-Caucasian, no. (%) | 55 (76%) | 50 (94%) | 47 (94%) | 7.3 | 0.007 | FET | 1.000 |
| Ever divorced (%) | 8 (11%) | 30 (57%) | 12 (24%) | 29.4 | <0.001 | 11.3 | 0.001 |
| Education, years, mean (SD) | 15.0 (2.2) | 13.8 (2.7) | 15.0 (2.4) | 8.8 | 0.003 | 7.6 | 0.006 |
| Educational achievement | 1.8, 3 | 0.614 | 9.4, 3 | 0.025 | |||
| Less than high school diploma, no. (%) | 5 (7%) | 7 (13%) | 2 (4%) | ||||
| High school diploma, no. (%) | 36 (50%) | 22 (42%) | 13 (26%) | ||||
| Associate’s degree/certificate, | 10 | 8 | |||||
| no. (%) | 12 (17%) | (19%) | (16%) | ||||
| Bachelor’s degree or higher, no. | 14 | 27 | |||||
| (%) | 19 (26%) | (26%) | (54%) | ||||
| Gambling preference | 44.1, 2 | <0.001 | |||||
| Action games1 | 53 (74%) | 8 (15%) | |||||
| Slots | 11 (15%) | 35 (66%) | |||||
| Other | 8 (11%) | 10 (19%) | |||||
| Mental health treatment past year, no. (%) | 18 (30%) | 15 (28%) | 6 (12%) | 0.0 | 0.843 | 4.2 | 0.040 |
| Psychiatric hospitalization, no. (%) | 12 (20%) | 13 (25%) | 2 (4%) | 0.3 | 0.563 | 8.7 | 0.003 |
| Treatment of PG, no. (%) | 19 (32%) | 28 (53%) | 0 (0%) | 5.2 | 0.023 | 36.3 | <0.001 |
| PG hospitalization, no. (%) | 2 (3%) | 9 (17%) | 0 (0%) | 6.1 | 0.013 | FET | 0.003 |
| # of PG DSM-IV criteria, mean (SD) | 8.0 (1.5) | 7.4 (1.6) | 3.5 | 0.063 | 3.5 | ||
| SOGS score, mean (SD) | 11.6 (4.3) | 11.1 (3.7) | 0.1 (0.4) | 0.4 | 0.516 | 84.1 | <0.001 |
| NODS score, mean (SD) | 8.1 (1.5) | 7.7 (2.0) | 0.0 (0.0) | 1.8 | 0.186 | 84.2 | <0.001 |
| BIS total score, mean (SD) | 67.2 (13.4) | 67.2 (9.9) | 55.8 (8.8) | 0.0 | 0.995 | 29.2 | <0.001 |
| BDI total score, mean (SD) | 10.6 (11.3) | 12.9 (10.4) | 4.7 (5.0) | 3.1 | 0.079 | 23.0 | <0.001 |
| Childhood maltreatment | |||||||
| Physical, no. (%) | 13 (21%) | 15 (31%) | 8 (20%) | 1.4 | 0.245 | 1.5 | 0.229 |
| Verbal, no. (%) | 24 (39%) | 21 (43%) | 10 (24%) | 0.2 | 0.659 | 3.4 | 0.066 |
| Sexual, no. (%) | 5 (8%) | 11 (22%) | 5 (12%) | 4.6 | 0.032 | 1.6 | 0.205 |
| Neglect, no. (%) | 6 (10%) | 6 (12%) | 4 (10%) | 0.2 | 0.665 | 0.1 | 0.708 |
| Emotional, no. (%) | 12 (20%) | 19 (40%) | 11 (27%) | 5.6 | 0.018 | 1.8 | 0.180 |
| Any maltreatment, no. (%) | 31 (50%) | 30 (61%) | 14 (34%) | 1.4 | 0.238 | 6.6 | 0.010 |
| Psychiatric comorbidity | |||||||
| Any substance use disorder, no. (%) | 47 (65%) | 29 (55%) | 10 (20%) | 1.4 | 0.232 | 13.2 | <0.001 |
| Alcohol use disorder, no. (%) | 39 (54%) | 28 (53%) | 9 (18%) | 0.0 | 0.882 | 13.6 | <0.001 |
| Drug use disorder, no. (%) | 30 (42%) | 3 (6%) | 2 (4%) | 20.4 | <0.001 | FET | 1.000 |
| Any anxiety disorder, no. (%) | 36 (50%) | 22 (42%) | 10 (20%) | 0.9 | 0.347 | 5.6 | 0.018 |
| GAD, no. (%) | 7 (10%) | 5 (9%) | 0 (0%) | 0.0 | 0.937 | FET | 0.057 |
| OCD, no. (%) | 12 (17%) | 2 (4%) | 0 (0%) | 5.1 | 0.024 | FET | 0.496 |
| Panic disorder, no. (%) | 12 (17%) | 8 (15%) | 3 (6%) | 0.1 | 0.813 | 2.2 | 0.135 |
| Specific phobia, no. (%) | 10 (14%) | 6 (11%) | 3 (6%) | 0.2 | 0.671 | FET | 0.490 |
| Social anxiety disorder, no. (%) | 10 (14%) | 5 (9%) | 3 (6%) | 0.6 | 0.449 | FE T | 0.716 |
| Any mood disorder, no. (%) | 40 (56%) | 31 (58%) | 17 (34%) | 0.1 | 0.743 | 6.2 | 0.013 |
| Bipolar disorder, no. (%) | 7 (10%) | 4 (8%) | 1 (2%) | 0.2 | 0.671 | FET | 0.364 |
| Major depression disorder, no. (%) | 37 (51%) | 27 (51%) | 15 (30%) | 0.0 | 0.961 | 4.7 | 0.031 |
| Any eating disorder, no. (%) | 4 (6%) | 7 (13%) | 1 (2%) | 2.2 | 0.136 | FET | 0.061 |
| Any impulse control disorder, | 10 | 0.10 | 0.00 | ||||
| no. (%) | 23 (32%) | (19%) | 1 (2%) | 2.7 | 1 | 7.7 | 6 |
| ADHD, no. (%) | 17 (24%) | 4 (8%) | 0 (0%) | 5.4 | 0.020 | FET | 0.118 |
| ASPD, no. (%) | 6 (9%) | 1 (2%) | 0 (0%) | 2.5 | 0.113 | FET | 1.000 |
| Past suicide attempt, no, (%) | 12 (17%) | 10 (19%) | 2 (4%) | 0.1 | 0.777 | 5.5 | 0.019 |
Action games include games that involve action or skill: cards, sports betting, video games, and horse or dog tracks
ASPD = antisocial personality disorder; ADHD = attention deficit/hyperactivity disorder; GAD = generalized anxiety disorder; OCD = obsessive-compulsive disorder, FET = Fisher’s Exact Test
Mean (SD) age at intake was 27.4 (7.7) years for younger PGs and 66.5 (6.8) years for older PGs. Mean (SD) age at PG onset was 22.1 (7.3) years for younger PGs and 50.0 (13.2) years for older PGs. Relative to younger PGs, older PGs were more likely to be female (64% vs. 19%), Caucasian (94% vs. 76%), and divorced (57% vs. 11%). Younger PGs preferred strategic (“action”) action games (74%), while older PGs preferred slots (66%). Older and younger PGs had similar rates of receiving mental health services in the past year and having a prior psychiatric hospitalization. Older PGs were more likely than younger PGs to have received PG treatment (53% vs. 32%) or to have been hospitalized for PG-related problems (17% vs. 3%). There were no significant differences between the groups on PG severity variables. Older PGs were more likely to report childhood sexual and emotional abuse. Several lifetime disorders were more common among younger than older PGs including drug use disorders (42% vs. 6%), obsessive-compulsive disorder (17% vs. 4%), and attention deficit/hyperactivity disorder (24% vs. 8%).
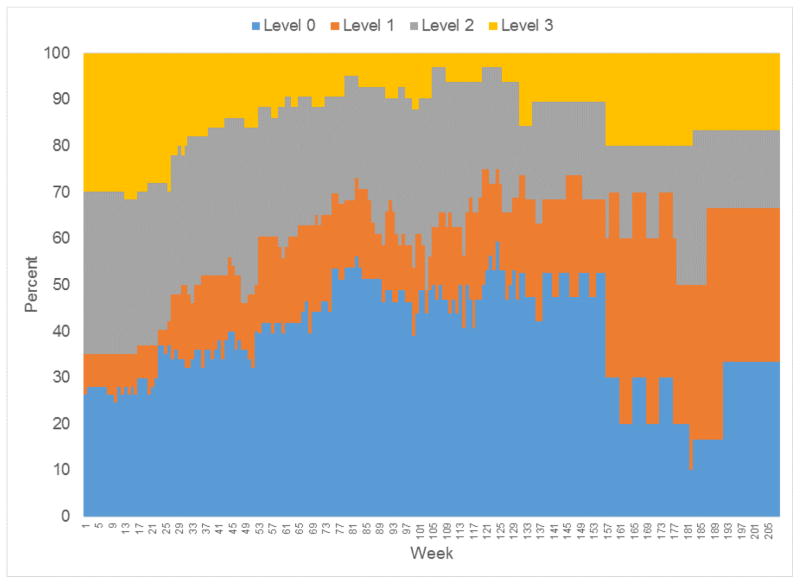
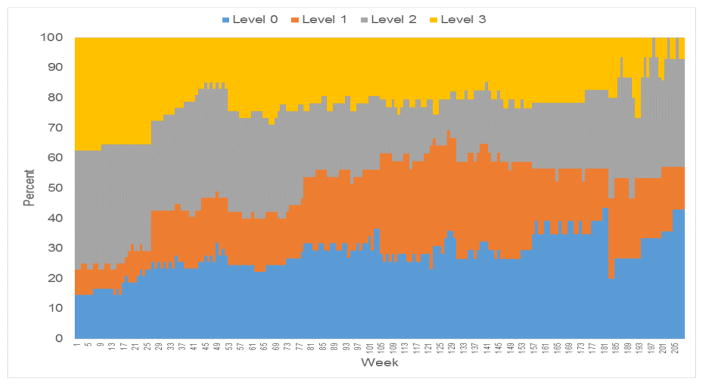
Gambling levels were assessed for 57 of the younger PGs, 48 of the older PGs, and all 50 of the elders without PG. Weekly measures of gambling activity are summarized in Figure 1 (younger PGs) and Figure 2 (older PGs). The relative frequency of gambling levels show greater variability over time because of decreasing sample size (Figures 1 and 2). For older PGs, the percentage achieving level 2 or 3 gambling peaked at 77% between baseline and week 14, and then decreased. The highest level of gambling activity for older PGs was level 3 for 38%, level 2 for 35%, level 1 for 4%, and level 0 for 13%. For younger PGs, the percentage with level 2 or 3 gambling peaked at 65% between baseline and week 16, and then decreased. The highest level of gambling activity was level 3 for 32% of the younger PGs, level 2 for 39%, level 1 for 7%, and level 0 (no gambling) for 12%.
Figure 1.
Weekly relative frequency of gambling activity levels for Younger PGs
Figure 2.
Weekly relative frequency of gambling activity levels for Older PGs
No older adult without PG developed PG (level 3) over the course of the follow-up. At-risk gambling (level 2) was not observed until week 82 for this group. At-risk gambling was observed intermittently between weeks 135 and 178; at week 160, two individuals (of 43) had at-risk gambling. The highest level of gambling activity was level 2 for 4%, level 1 for 40%, and level 0 for 56%.
There were no significant differences in PG outcomes between younger and older PGs (Table 2). On average, younger PGs spent 28.5% of the follow-up in remission, 16.6% in partial remission, and 45.3% in chronic PG. On average, older PGs spent 21.9% of the follow-up in remission, 19.9% in partial remission, and 49.3% in chronic PG. The percentage of weeks showing a change in gambling activity (from the previous week) was similar for younger (4.4%) and older PGs (5.0%). On average, elders without PG spent 78.5% of the follow-up with no gambling activity and 20.7% of the follow-up engaged in recreational gambling. Among the 105 PGs with follow-up data, 52 (49.5%) had periods of remission. Among these individuals, time to relapse was similar for older and younger PGs (Cox Proportional Hazards Chi-Square < 0.1, df = 1, P = 0.833).
Table 2.
PG course outcomes for younger PGs, older PGs, and elder controls
| PG course outcome | % Weeks, Mean (SD)
|
Older vs.Younger PGs
|
|||
|---|---|---|---|---|---|
| Younger PGs (n=57) | Older PGs (n=48) | Elder Controls (n=50) | |||
| OR (95% C.I.) | P-value | ||||
| Level 2 or 3 | 49.8 (41.4) | 53.5 (39.9) | 0.3 (1.9) | 1.28 (0.69, 2.37) | 0.442 |
| Level 3 | 20.4 (32.8) | 24.1 (31.8) | 0.0 (0.0) | 1.42 (0.69, 2.91) | 0.346 |
| Remission | 28.5 (36.7) | 21.9 (36.4) | 78.5 (33.8) | 0.68 (0.32, 1.45) | 0.322 |
| Partial remission | 16.6 (24.8) | 19.9 (21.9) | 20.7 (32.5) | 1.16 (0.56, 2.40) | 0.690 |
| Remission or partial remission | 41.7 (38.0) | 39.8 (38.4) | 94.0 (6.7) | 0.94 (0.55, 1.61) | 0.813 |
| Chronic PG | 45.3 (41.5) | 49.3 (40.5) | 0.0 (0.0) | 1.29 (0.70, 2.36) | 0.419 |
| Change in gambling activity level | 4.4 (5.8) | 5.0 (8.8) | 1.2 (2.4) | 0.86 (0.49, 1.50) | 0.593 |
Decreased gambling activity during follow-up was observed in younger and older PGs (Table 3). The odds of problem gambling (levels 2 and 3 combined) decreased by a factor of 0.65 per year of follow-up for both groups. Similarly, the odds of remission or partial remission increased by a factor of 2.26 for younger PGs (95% CI, 1.70, 3.02), and by a factor of 1.99 for older PGs (95% CI, 1.64, 2.41). The odds of chronic PG decreased significantly over time for both groups, and the odds of any change in gambling activity level increased for younger PGs (OR = 1.23, 95% CI, 1.05, 1.44). None of the slopes measuring change in the PG course outcomes varied significantly for younger and older PGs.
Table 3.
Changes in PG course outcomes for younger PGs and older PGs
| PG course outcome | Odds Ratio per one year of follow-up (95% C.I.) | Test of slope difference | ||
|---|---|---|---|---|
|
| ||||
| Younger PGs (n=57) | Older PGs (n=48) | Z | P-value | |
| Level 2 or 3 | 0.65 (0.49, 0.86) | 0.65 (0.53, 0.79) | −0.07 | 0.944 |
| Level 3 | 0.60 (0.37, 0.99) | 0.68 (0.54, 0.86) | 0.44 | 0.660 |
| Remission | 1.21 (0.97, 1.51) | 1.33 (1.11, 1.59) | 0.67 | 0.500 |
| Partial remission | 1.42 (1.04, 1.94) | 1.41 (1.16, 1.71) | −0.04 | 0.968 |
| Remission or partial remission | 2.26 (1.70, 3.02) | 1.99 (1.64, 2.41) | −0.73 | 0.463 |
| Chronic PG | 0.57 (0.42, 0.76) | 0.63 (0.51, 0.77) | 0.53 | 0.597 |
| Change in gambling activity level | 1.23 (1.05, 1.44) | 1.22 (0.90, 1.65) | −0.04 | 0.971 |
4. Discussion
Results showed that gambling activity oscillates with those at level 2 and 3 moving toward an overall reduction of gambling activity. We confirmed our hypothesis that PGs become less symptomatic or remit during follow-up, but were unable to show that older adults with PG had greater improvement than younger PGs. In fact, both groups experienced similar levels of improvement. The data challenge the notion that PG is intractable
Data shown in Figures 1 and 2 are largely consistent with research findings that suggest PGs experience a gradual diminution of gambling severity over time (Shaffer and Hall, 2002; Winters et al., 2002; Slutske et al., 2003; Abbott et al., 2004; DeFuentes-Merillas et al., 2004; LaPlante et al., 2008; Edgerton et al., 2015). The findings were true for younger and older PGs, though a substantial percentage of PGs remained at level 3 gambling during follow-up. For these individuals, PG is chronic but not necessarily progressive. Nearly 40% in each group experienced a partial of full remission during follow-up. Importantly, the data suggest that lifetime PGs already in remission or who gamble recreationally will maintain these gains and not worsen.
The view that PG is chronic and progressive was promoted by Custer (1985) who described PG as a multi-stage illness that began with a winning phase, followed by a losing phase, and ending in a desperation phase. Custer’s views were primarily informed by his clinical experience and gained wide acceptance despite the lack of supporting data. A review by LaPlante et al. (2008), and other work (Sartor et al., 2008; Edgerton et al., 2015), have gradually contributed to a more nuanced view of PG course. LaPlante et al. reviewed five studies that met their criteria of reporting follow-up data pertaining to gambling that did not involve a treatment sample (Shaffer and Hall, 2002; Winters et al., 2002; Slutske et al., 2003; Abbott et al., 2004; DeFuentes-Merillas et al., 2004). Briefly, level 3 gamblers improved with most moving to a lower gambling level, with similar results for level 2 gamblers. Level 0–1 gamblers at baseline were unlikely to progress to a higher (i.e., more severe) level of gambling behavior. Thus, most PGs and at-risk gamblers moved to lower levels of gambling involvement over time, while those who gamble recreationally (or did not gamble) were unlikely to move to a more severe level of gambling activity.
More recently, several population-based studies have examined the trajectory of problem and pathological gambling. Edgerton et al. (2015) followed 679 young adults over four years and found a pattern of lessening, and not worsening gambling severity scores. In the Quinte Study of Gambling and Problem Gambling, Williams et al. (2015) followed 4,121 adults to assess problem behaviors and found that problem gamblers and PGs moved toward lower levels of gambling involvement over 5 years. Finally, in a 5-year longitudinal study of 1808 children and adults (Leisure, Lifestyle and Lifecycle Project), el-Guebaly et al. (2015) reported that there was a considerable amount of transition at the individual level, with 80% of adults with problem gambling having at least one year of remission with only a third relapsing.
Our data also confirm important differences between the study groups. PG groups were more symptomatic than older adults without PG in terms of trait impulsiveness, depressive symptoms, and co-occurring disorders. These differences were expected and not surprising. Of note, there were no differences between the two PG groups in overall gambling severity or trait impulsiveness. We had expected that trait impulsiveness would be higher in younger subjects. We based this expectation in part on the observation that they were significantly more likely to drop out or become lost to follow-up, and because of our earlier work comparing younger- and older-onset PGs found that younger PGs scored significantly higher on impulsiveness (Black et al., 2015).
The comparison between younger and older PGs showed many differences. Older PGs were more likely to be women, Caucasian, divorced, and to have a lower level of education. Older PGs were more likely to have sought mental health treatment, to have been psychiatrically hospitalized, and to have sought PG treatment. Some of these findings may have resulted from the fact that older PGs simply had more time to seek treatment and to have failed at marriage, leading to divorce. Likewise, older PGs may have been more likely to seek treatment because women tend to seek mental health treatment at higher rates than men (Kessler et al., 1981). Older PGs were also more likely to report a history of childhood maltreatment (e.g., sexual abuse, emotional abuse). Several of the findings were close to achieving significance (P < 0.05), such as total BDI score (older PGs had higher scores) and “any impulse control disorder” (younger PGs had more ICDs), but the results were not significant possibly due to low statistical power.
Older PGs had lower rates of many lifetime comorbid disorders than younger PGs including drug use disorders, attention deficit/hyperactivity disorder, and obsessive-compulsive disorder. We had expected younger PGs to have higher rates of substance misuse and attention deficit/hyperactivity disorder based on prior work by our group (Black et al., 2013; 2014), and the fact that these disorders tend to be more common in younger persons in the general population. The finding of higher rates of obsessive-compulsive disorder in younger PGs was unexpected and its importance is not immediately clear. That older PGs preferred slots and the younger PGs preferred action games were findings also consistent with earlier work by our group and others (Vizcaino et al., 2013; Black et al., 2015).
The differences we observed between older and younger PGs appear to partially validate two classification schemes that have been proposed. The most widely held scheme is the “escape-seeking” vs. “sensation-seeking” gamblers. Escape-seeking persons gamble to relieve emotional tension, anxiety, or depression (Blasczcynski and McConaghy, 1989). For such people, gambling may provide an escape from unpleasant affects. In contrast, sensation-seeking persons seek stimulation and arousal to relieve boredom or hyperarousal. Considering their “pathways” model (Blaszczynski and Nower, 2002), our younger PGs have many similarities with their “antisocial, impulsive gambler.” These individuals are mostly male, and tend to start gambling early in life, rapidly developing problematic gambling. They appear to respond to the excitement and stimulation that gambling often provides. Our older PGs better fit their description of the “emotionally vulnerable gambler.” These individuals tend to be women with depression or anxiety, have difficulty coping, and are more likely to experience childhood maltreatment. For them, gambling serves to modulate mood states or meet other psychological needs.
There are several methodologic limitations to acknowledge. First, PG subjects recruited through an epidemiological sampling method may have been desirable, but this was not feasible. Second, older adult controls were identified through a random digit dialing method, and it is possible that they may not be representative because many people no longer use land lines. Third, follow-up interviews were conducted by telephone, and it is possible that in-person interviews could provide more information. Fourth, the low rate of participation of minority subjects reduces the generalizability of our findings in these populations. Fifth, a larger sample with longer follow-up would have been preferred, but budgetary constraints precluded those options. Sixth, because our sample sizes were relatively small, statistical power may have been compromised. For example, several comparisons in Table 1 appeared clinically meaningful (e.g., total BDI score) and might have been significant with larger numbers of subjects, Also, because of the potential for Type II errors, and the study is exploratory, we chose not to control for the many comparisons. We concluded that a Bonferroni correction would have been overly conservative. Seventh, nearly 30% of subjects discontinued participation, and while we replaced those who dropped out, attrition could have led to a systematic bias because those who dropped out were more likely to be younger. Last, it is possible that the “Hawthorn effect” may have contributed to improvement in those with PG (McCambridge et al., 2014). The Hawthorn effect posits that subjects who are aware of being observed will alter their behavior. Despite these limitations, the fact that our findings are consistent with work by other investigators suggests they are valid (Slutske et al., 2003; LaPlante et al., 2008).
Additional follow-up studies with careful tracking of gambling behavior are warranted to better understand the course of PG. Follow-up interviews at frequent intervals may improve participation, reduce attrition, and minimize “memory problems” inherent in longitudinal studies thereby leading to more accurate reporting.
5. Conclusion
In a longitudinal study of older and younger persons with PG, week by week gambling activity levels showed a significant general downward movement for all subjects, and there were no significant differences between the groups. Older adults without PG had no change in their level of gambling activity. We conclude that PGs move toward a reduced level of gambling activity during follow-up. Our data challenge the notion that PG is chronic and progressive.
Highlights.
Seventy-two younger (<40 years) and 50 older (≥ 60 years) subjects with pathological gambling (PG), and 53 older adults without PG were systematically interviewed every 6 months to assess gambling behavior and other variables. Mean follow-up was 2.6 years.
Older PGs were more likely to be female, Caucasian, divorced, and to have a lower level of education. Older and younger PGs were similar in severity, but older PGs were more likely to have sought PG treatment.
Older PGs had lower rates of lifetime drug use disorders, attention deficit/hyperactivity disorder, and obsessive-compulsive disorder. They preferred slots, had worse physical health ratings, and were less likely to discontinue participation.
Week by week gambling activity levels showed a significant general downward movement for older and younger PGs, although there were no differences between the groups. Older adults without PG had no change in their level of gambling activity.
Younger and older PGs moved toward a reduced level of gambling activity during the follow-up. Our data challenge the notion that PG is chronic and progressive.
Acknowledgments
The research was supported through a grant from the National Institute on Aging (RO1AG037132), Bethesda, MD (Dr. Black). Dr. Black receives royalties from American Psychiatric Publishing, Oxford University Press, and UpToDate. Drs. Coryell and Allen, Ms. Shaw and Mr. McCormick report no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MW, Williams MM, Volberg RA. A prospective study of problem and regular non-problem gamblers living in the community. Substance Use and Misuse. 2004;39:855–884. doi: 10.1081/ja-120030891. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Press; Washington, DC: 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Barratt E. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9:191–198. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio: Psychological Corp; 1996. [Google Scholar]
- Black DW, Grant JE. DSM-5 Guidebook: The Essential Companion to the Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; Washington, DC: 2014. [Google Scholar]
- Black DW, Smith M, Forbush KT, Shaw M, Moser D, Allen J. Neuropsychological performance, impulsivity, symptoms of ADHD, and Cloninger’s personality traits, in pathological gambling. Addiction Research and Therapy. 2013;21:216–226. [Google Scholar]
- Black DW, Coryell WC, Crowe RR, McCormick B, Shaw M, Allen J. A direct, controlled, blind family study of pathological gambling. Journal of Clinical Psychiatry 2014. 2014;75:215–221. doi: 10.4088/JCP.13m08566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Coryell WH, Crowe RR, Shaw M, McCormick B, Allen J. Age at onset of DSM-IV pathological gambling in a non-treatment sample: early-versus later-onset. Comprehensive Psychiatry. 2015;60:40–46. doi: 10.1016/j.comppsych.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Arndt S, Coryell WH, Argo T, Forbush KT, Shaw M, Perry P, Allen J. Bupropion in the treatment of pathological gambling: a randomized, placebo-controlled, flexible-dose study. Journal of Clinical Psychopharmacology. 2007;27:143–150. doi: 10.1097/01.jcp.0000264985.25109.25. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, McConaghy N. Anxiety and/or depression in the pathogenesis of addictive gambling. International Journal of Addictions. 1989;24:337–350. doi: 10.3109/10826088909047292. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Nower L. Pathways model of problem and pathological gambling. Addiction 2002. 2002;97:487–499. doi: 10.1046/j.1360-0443.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Christenson GA, Faber RJ, de Zwaan M, Raymond NC, Specker SM, Ekern MD, Mackenzie TB, Crosby RD, Crow SJ, Eckert ED. Compulsive buying: Descriptive characteristics and psychiatric comorbidity. Journal of Clinical Psychiatry. 1994;55:5–11. [PubMed] [Google Scholar]
- Clark L. In: Epidemiology and phenomenology of pathological gambling in The Oxford Handbook of Impulse Control Disorders. Grant JE, Potenza MN, editors. Oxford University Press; New York: 2012. pp. 94–116. [Google Scholar]
- Custer R. When Luck Runs Out. New York: Facts on File; 1985. [Google Scholar]
- DeFuentes-Merillas L, Koeter MW, Schippers GM, van den Brink W. Temporal stability of pathological scratchcard gambling among adult scratchcard buyers two years later. Addiction. 2004;99:117–127. doi: 10.1111/j.1360-0443.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- Edgerton JD, Melnyk TS, Roberts LW. Problem gambling and the youth-to-adulthood transition: assessing problem gambling severity trajectories in a sample of young adults. Journal of Gambling Studies. 2015;31:1463–1485. doi: 10.1007/s10899-014-9501-2. [DOI] [PubMed] [Google Scholar]
- el-Guebaly N, Casey DM, Currie SR, Hodgins DC, Shopflocher DP, Smith GJ, Williams RJ. Final Report to the Alberta Gambling Research Institute. Alberta, Canada: 2015. The Leisure, Lifestyle, & Lifecycle Project (LLLP): A Lpngitudinal Study of Gambling in Alberta. [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Erickson L, Molina CA, Ladd GT, Pietrzak RH, Petry NM. Problems and pathological gambling are associated with poorer mental and physical health in older adult. International Journal of Geriatric Psychiatry. 2005;20:754–759. doi: 10.1002/gps.1357. [DOI] [PubMed] [Google Scholar]
- Goskar E. The marketing of gambling to the elderly. Elder Law Journal. 1999;7:185–216. [Google Scholar]
- Granero R, Penelo E, Stinchfield R, Fernandez-Aranda F, Savvidou LG, Froberg F, Aymami N, Gomez-Pena M, Perez-Serrano M, del Pino-Guiterrez A, Menchon JM, Jimenez-Murcia S. Is pathological gambling moderated by aging? Journal of Gambling Studies. 2014;30:475–492. doi: 10.1007/s10899-013-9369-6. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Brown E. Characteristics of geriatric patients seeking medication treatment for pathologic gambling disorder. Journal of Geriatric Psychiatry and Neurology. 2001;14:125–129. doi: 10.1177/089198870101400305. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL, Buchanan SN, Potenza MN. Late-onset pathological gambling: clinical correlates and gender differences. Journal of Psychiatric Research. 2009;43:380–387. doi: 10.1016/j.jpsychires.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Brown RL, Broman CL. Sex differences in psychiatric help-seeking: evidence from four large-scale surveys. Journal of Health and Social Behavior. 1981;22:49–62. [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorder: a review of the recent lilterature. Current Opinion in Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biological Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Lader M. Antiparkinsonian medication and pathological gambling. CNS Drugs. 2008;22:407–416. doi: 10.2165/00023210-200822050-00004. [DOI] [PubMed] [Google Scholar]
- LaPlante DA, Nelson SE, LaBrie RA, Shaffer HJ. Stability and progression of disordered gambling: lessons from longitudinal studies. Canadian Journal of Psychiatry. 2008;53:52–60. doi: 10.1177/070674370805300108. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB The South Oaks Gambling Screen (SOGS) A new instrument for identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Loraines FK, Cowlishaw S, Thomas SA. Prevalence of comorbid disorders in problem and pathological gambling: systematic review and meta-analysis of population surveys. Addiction. 2011;106:490–498. doi: 10.1111/j.1360-0443.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- McCambridge JW, Witton J, Elbourne DR. Systematic review of the Hawthorn effect: new concepts are needed to study research participation effects. Journal of Clinical Epidemiology. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Opinion Research Center at the University of Chicago (NORC) Report to the National Gambling Impact Study Commission. 1999. Gambling Impact and Behavior Study. [Google Scholar]
- McNeilly DP, Burke WJ. Gambling as a social activity of older adult. International Journal of Aging and Human Development. 2001;52:19–28. doi: 10.2190/A4U7-234X-B3XP-64AH. [DOI] [PubMed] [Google Scholar]
- Pavalko RM. In: Problem gambling among older people in Treating Alcohol and Drug Abuse in the Elderly. Gurnack AM, Atkinson RM, Osgood NJ, editors. Springer; New York: 2002. pp. 190–213. [Google Scholar]
- Pfohl B, Zimmerman M, Blum N. A Structured Interview for DSM-IV Personality Disorders (SIDP-IV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Pilver CE, Potenza MN. Increased incidence of cardiovascular conditons among older adutls with pathological gambling features in a prospective study. Journal of addiction Medicine. 2013;7 doi: 10.1097/ADM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Scherrer JF, Shah KR, Xian H, Volberg R, Eisen SA. Course of pathological gambling symptoms and reliability of the Lifetime Gambling History measure. Psychiatry Research. 2007;152:55–61. doi: 10.1016/j.psychres.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN. Estimating the prevalence of adolescent gambling disorders: a quantitative synthesis and guide toward standard gambling nomenclature. Journal of Gambling Studies. 1996;12:193–214. doi: 10.1007/BF01539174. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN. The natural history of gambling and drinking problems among casino workers. Journal of Social Psychology. 2002;142:405–424. doi: 10.1080/00224540209603909. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan KH, Amorim O, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini International Neuropsychiatric Interview (MINI) Journal of Clinical Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Slutske WS. Natural recovery and treatment seeking in pathological gamboing: results of two U.S. National surveys. American Journal of Psychiatry. 2006;163:297–302. doi: 10.1176/appi.ajp.163.2.297. [DOI] [PubMed] [Google Scholar]
- Slutske W, Jackson KM, Sher KJ. The natural history of problem gambling from age 18 to 29. Journal of Abnormal Psychology. 2003;112:263–274. doi: 10.1037/0021-843x.112.2.263. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Time Line Follow back User’s Manual. Addiction Research Foundation; Toronto: 1995. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbons M. Structured Clinical Interview for DSM-IV. New York State Psychiatric Institute, Biometrics Research; New York: 1994. [Google Scholar]
- Tavares H, Martins SS, Lobo DSS, Silviera CM, Gentil V, Hodgins DC. Factors at play in faster progression for female pathological gamblers: an exploratory analysis. Journal of Clinical Psychiatry. 2003;64:433–438. doi: 10.4088/jcp.v64n0413. [DOI] [PubMed] [Google Scholar]
- Vizcaino EJV, Fernandez-Navarro P, Petry N, Rubio G, Blanco C. Differences between early-onset pathological gambling and later-onset gambling: data from the National Epidemiologic Survey on Alcohol and Related Disorders (NESARC) Addiction. 2013;109:807–813. doi: 10.1111/add.12461. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. New England Medical Center Health Institute; Boston: 1993. [Google Scholar]
- Warshaw MG, Keller MB, Stout RL. Reliability and validity of the Longitudinal Interval Follow-up Evaluation for assessing outcome in anxiety disorders. Journal of Psychiatric Research. 1994;28:531–545. doi: 10.1016/0022-3956(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Welte J, Barnes G, Wieczorek W, Tidwell MC, Parker J. Alcohol and gambling pathology among U.S. adults: prevalence, demographic patterns and comorbidity. Journal of Studies on Alcohol. 2001;62:706–712. doi: 10.15288/jsa.2001.62.706. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Hann R, Shopflocher D, West B, McLaughlin P, White N, King K, Flexhaug T. Report prepared for the Ontario Problem Gambling Research Center. Ontario, Toronto: 2015. Quinte Longitudinal Study of Gambling and Problem Gambling. [Google Scholar]
- Winters KC, Stinchfield RD, Botzet A, Anderson N. A prospective study of youth gambling behaviors. Psychology of Addictive Behavior. 2002;16:3–9. doi: 10.1037//0893-164x.16.1.3. [DOI] [PubMed] [Google Scholar]
- Wohl MJA, Sztainert T. Where did all the pathological gamblers go? Gambling symptomatology and stage of change predict attrition in longitudinal research. Journal of Gambling Studies. 2011;27:155–169. doi: 10.1007/s10899-010-9186-0. [DOI] [PubMed] [Google Scholar]