Abstract
Background
We hypothesized that clonidine and propranolol would increase VEGF and VEGF-receptor expression and promote lung healing following severe trauma and chronic stress.
Methods
Sprague-Dawley rats were subjected to lung contusion (LC), lung contusion/hemorrhagic shock (LCHS), or lung contusion/hemorrhagic shock/daily restraint stress (LCHS/CS). Clonidine and propranolol were administered daily. On day seven, lung VEGF, VEGFR-1, VEGFR-2, and HMGB1 were assessed by PCR. Lung injury was assessed by light microscopy (*p<0.05).
Results
Clonidine increased VEGF expression following LCHS (43%*) and LCHS/CS (46%*). Clonidine increased VEGFR-1 and R-2 expression following LCHS/CS (203%* and 47%*, respectively). Clonidine decreased HMGB1 and TNF-alpha expression following LCHS/CS (22%* and 58%*, respectively.) Clonidine decreased inflammatory cell infiltration and total Lung Injury Score following LCHS/CS. Propranolol minimally affected VEGF and did not improve lung healing.
Conclusions
Clonidine increased VEGF and VEGF-receptor expression, decreased HMGB1 expression, decreased lung inflammation, and improved lung tissue repair.
Keywords: Vascular endothelial growth factor, trauma, stress, lung contusion, tissue repair
Introduction
Vascular endothelial growth factor (VEGF) plays an important role in restoring homeostasis following lung injury by propagating angiogenesis, potentiating pulmonary endothelial cell growth and survival, and stimulating type II alveolar cell surfactant production.1, 2 Animal studies have shown that following traumatic lung injury, VEGF and VEGF receptor expression increase over the course of seven days, and there is no histologic evidence of lung injury one week after injury.3 However, when lung contusion (LC) is followed by hemorrhagic shock (HS), VEGF and VEGF receptor expression are decreased, and lung tissue does not heal.3 The addition of chronic stress (CS) to LC and HS further suppresses VEGFR-1, and is associated with greater lung injury. These findings in animal studies are similar to data from human Acute Respiratory Distress Syndrome (ARDS) patients, for whom increased VEGF levels have been associated with resolution of lung injury.4 Therefore, restoration of normal VEGF and VEGF receptor expression may be therapeutic for severely injured blunt trauma patients who are subjected to uncontrolled hemorrhage and the daily stressors of the intensive care unit environment.
The purpose of this study was to investigate the effects of two medications that modulate the neuroendocrine stress response, clonidine and propranolol, on VEGF and VEGF receptor expression and lung tissue repair following LC, HS and CS. Propranolol blocks the MAPK, JNK, and NF-κB stress response pathways occurring downstream of the NE-beta adrenergic receptor complex, and improves burn wound healing.5–8 Clonidine is also known to attenuate the inflammatory response following surgery and experimental sepsis, though it has not been studied extensively in would healing and tissue repair.9, 10 Differences in mechanism of action between these medications may also elucidate the relative importance of central sympathetic tone and peripheral adrenergic receptors in stress-mediated would healing dysfunction. Clonidine reduces central sympathetic tone by stimulating alpha-2 adrenergic receptors in the medulla oblongata and reduces NE release, whereas propranolol has the ability to competitively block the actions of epinephrine and norepinephrine at peripheral beta-adrenergic receptors.11, 12 We hypothesized that daily clonidine or propranolol administration in a rodent model of lung contusion/hemorrhagic shock with chronic restraint stress would increase VEGF and VEGF receptor expression, and that these effects would be associated with lung tissue healing.
Methods
Eight week-old male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 300–400g were housed in pairs and fed ad lib with Teklad Diet #7912 (Harlan Laboratories Inc., Tampa, FL) and water for a one week acclimation period. Dark and light cycles were 12 hours each during acclimation and experimental periods. All animal care was conducted in accordance with the Institutional Animal Care and Use Committee standards. Animals were randomly allocated ten different groups (n = 6–8 per group): 1) naïve control, 2) lung contusion (LC), 3) LC with clonidine, 4) LC with propranolol, 5) lung contusion followed by hemorrhagic shock (LCHS), 6) LCHS with clonidine, 7) LCHS with propranolol, 8) lung contusion followed by hemorrhagic shock and daily restraint stress (LCHS/CS), 9) LCHS/CS with clonidine, 10) LCHS/CS with propranolol. Prior to the initial injury, animals were anesthetized by intraperitoneal (IP) injection of sodium pentobarbital (50 mg/kg). LC was performed by applying a percussive staple gun (PowerShot Model 5700M, Saddle Brook, NJ) to a 12 mm copper plate applied to the right lateral chest wall 1 cm below the axillary crease. This model has previously been shown to produce a clinically significant and reproducible pulmonary contusion.13–15
Rats allocated to HS groups were then placed on a heating pad, and the right internal jugular vein and right femoral artery were cannulated under direct visualization. Continuous blood pressure monitoring was performed by securing the arterial catheter to a BP-2 Digital Blood Pressure Monitor (Columbus Instruments, Columbus, OH). Blood was then withdrawn through the venous catheter into a heparinized syringe until a mean arterial pressure of 30–35 mm Hg was obtained. This blood pressure was maintained for a 45-minute period by withdrawing or reinfusing blood as necessary. After 45 minutes of hemorrhagic shock, blood was reinfused at 1 mL/min. Animals did not receive intravenous or subcutaneous fluids at any point.
CS was performed by placing animals in a restraint cylinder (Kent Scientific Corporation, Torrington, CT) for two hours daily. CS began one day after LCHS in the LCHS/CS group. In order to prevent acclimation to the restraint cylinder, the cylinders were rotated 180 degrees every 30 minutes, and alarms and sirens (80 dB) were transmitted by speakers placed immediately adjacent to the cylinders for two minutes each time the cylinders were rotated. All non-CS groups were subjected to a two hour daily fast while CS was administered.
Clonidine and propranolol were administered by intraperitoneal injection 10 minutes following resuscitation from hemorrhagic shock, and then daily following CS or daily handling. Clonidine and propranolol doses were 75 μg/kg and 10 mg/kg, respectively, based on previous work demonstrating the safety and efficacy of these doses in reducing heart rate by 10–20% without causing significant hypotension.13, 16 Propranolol and clonidine were administered once daily rather than more frequent dosing because the goal was to attenuate the neuroendocrine stress response following injury and daily restraint stress rather than to maintain a steady state of pharmacotherapy. Because norepinephrine has a short half-life, a single dose of propranolol or clonidine following resuscitation from hemorrhagic shock or cessation of restraint stress was given.
Animals were sacrificed by cardiac puncture following IP injection of ketamine (80–100 mg/kg) and xylazine (5–10 mg/kg) on day seven. Right lung and plasma specimens were collected. Lung specimens were initially placed in phosphate buffered saline (PBS). One portion of the contused right lung was placed in formalin for hematoxylin and eosin staining and histologic analysis by light microscopy, and another portion was placed immediately in dry ice and then stored at −80°C. Plasma samples were obtained during cardiac puncture by withdrawing 7–10 mL of blood into a heparinized syringe. Blood was then centrifuged at 500G for 5 minutes and plasma was aliquoted, placed immediately in dry ice, and then stored at −80°C.
Lung VEGF, VEGFR-1, VEGFR-2, high mobility group box 1 (HMGB1), and tumor necrosis factor alpha (TNF-alpha) expression were assessed by endpoint polymerase chain reaction. HMGB1 expression was measured as a molecular indicator of lung inflammation to correspond with histological findings. The following primers were selected: VEGF forward 5′ gtggacttgagttgggagga and reverse 5′ caaacagacttcggcctctc (product region: 2135–2228, product size: 147 bp), VEGFR-1 forward 5′ agtggctccacgaccttaga and reverse 5′ gaagaccgcttcagttttcgt (product region: 2258–2575, product size: 317 bp), VEGFR-2: forward 5′ acagcatcaccagcagtcag and reverse 5′ ccaagaactccatgccctta (product region: 3127–3274, product size: 147 bp), HMGB1 forward 5′ gttctgagtaccgcccaaaa and reverse 5′ ttcatcctcctcgtcgtctt (product region: 374–639, product size: 264 bp), TNF-alpha forward 5′ gaaacacacgagacgctgaa and reverse 5′ ccagatggggatagctggta (product region: 1034–1485, product size: 452 bp). Amplifications were performed using a SimpliAmp thermal cycler (Applied Biosystems, Carlsbad, CA) with an initial 4 minute denaturation phase at 95°C, followed by 32 cycles with denaturation at 95°C, annealing at 60°C, and extension at 72°C for 45 seconds each. Products were separated on 1.5% agarose gel stained with Ethidium Bromide (Invitrogen, Carlsbad, CA). TNF-alpha and HMGB1 were measured on day seven to quantify the persistent inflammatory state following severe trauma and chronic stress to assess if chronic stress was associated with persistent inflammation and impaired wound healing.
Lung injury was assessed by a board certified pathologist using light microscopy to visualize portions of the contused right lung stained with hematoxylin and eosin. The pathologist was blinded to experimental group allocation. The severity of lung injury was analyzed according to the LIS scoring system described in Table 1, adapted from Claridge et al.17 and Matute-Bello et al.18
Table 1.
Lung Injury Score calculator adapted from Claridge et al.17 and Matute-Bello et al.18 HPF: high-power field.
| Components | Points |
|---|---|
| Inflammatory cells/HPF | |
| <5 | 0 |
| 6 to 10 | 1 |
| 11 to 15 | 2 |
| 16 to 20 | 3 |
| >20 | 4 |
|
| |
| Interstitial edema | |
| None | 0 |
| Minimal | 1 |
| Moderate | 2 |
| Severe | 3 |
|
| |
| Pulmonary edema | |
| <5% | 0 |
| 5–25% | 1 |
| >25% | 2 |
|
| |
| Alveolar integrity | |
| Normal | 0 |
| Moderately abnormal | 1 |
| Severely abnormal | 2 |
Statistical analysis was performed using GraphPad Prism (version 6.05, GraphPad Software, La Jolla, CA) to calculate one-way analysis of variance. Data were reported as mean ±standard deviation with α = 0.05.
Results
The Effects of Clonidine on VEGF and VEGF Receptors
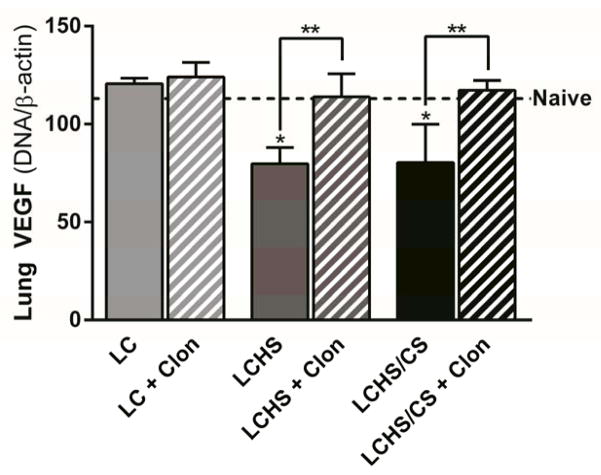
There was no change in lung VEGF expression following LC only or LC followed by clonidine administration (Figure 1). Seven days following LCHS and LCHS/CS, lung VEGF expression was decreased (Figure 1). With clonidine administration, lung VEGF expression was restored to naïve levels (LCHS + clonidine: 43% increase, p = 0.003; LCHS/CS + clonidine: 46% increase, p = 0.006; Figure 1).
Figure 1.
Clonidine increased lung VEGF expression seven days following LCHS and LCHS/CS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
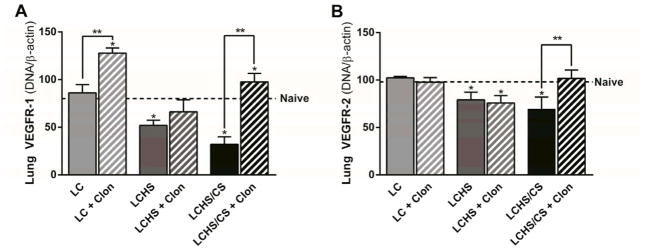
Lung VEGFR-1 expression did not change seven days after LC when compared to naïve animals (Figure 2A). Lung VEGFR-1 expression was decreased following LCHS and LCHS/CS when compared to naïve animals (Figure 2A). Administration of clonidine increased VEGFR-1 expression following LC, LCHS, and LCHS/CS, though the difference was not statistically significant in the LCHS group (LC + clonidine: 61% increase, p = 0.01; LCHS + clonidine: 28% increase, p = 0.131; LCHS/CS + clonidine: 203% increase, p = 0.001).
Figure 2.
Figure 2A. Clonidine increased lung VEGFR-1 expression seven days following LC and LCHS/CS. 2B: Clonidine increased lung VEGFR-2 expression seven days following LCHS/CS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
Lung VEGFR-2 expression did not change following LC or LC followed by clonidine administration. Lung VEGFR-2 expression was suppressed following LCHS and LCHS/CS, and was significantly increased among LCHS/CS animals that received clonidine, but slightly decreased among LCHS animals that received clonidine when compared to untreated counterpart (LCHS + clonidine: 4% decrease, p = 0.600; LCHS/CS + clonidine: 47% increase, p = 0.004; Figure 2B).
The Impact of Clonidine on Lung Injury Scores
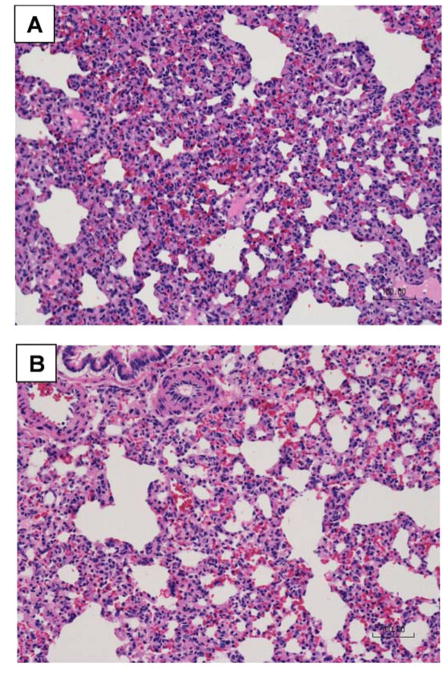
Following LC alone, total LIS is 0.8 indicating that the injured lung has healed on day seven. Clonidine administration significantly decreased inflammatory cell infiltration (2.4* vs. 3.8, p = 0.011) and total LIS (5.4* vs. 8.2, p = 0.020) in LCHS/CS animals when compared to LCHS alone (Table 2). Clonidine administration following LCHS and LCHS/CS was not associated with significant differences in interstitial edema, pulmonary edema, or alveolar integrity (Table 2). Figure 3 includes representative histologic samples comparing LCHS/CS (Figure 3A) to LCHS/CS followed by clonidine administration (Figure 3B) illustrating differences in the number of inflammatory cells and overall lung injury severity.
Table 2.
Clonidine improved lung tissue repair following LCHS/CS. LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, ap = 0.011 vs. LCHS/CS, bp = 0.020 vs. LCHS/CS. Data are reported as mean ±standard deviation.
| Components | LCHS | LCHS + Clonidine | LCHS/CS | LCHS/CS + Clonidine |
|---|---|---|---|---|
| Inflammatory cells/HPF (naïve: 0.3 ± 0.5) | 3.3 ±0.5 | 3.0 ±1.3 | 3.8 ±0.4 | 2.4 ±0.9a |
| Interstitial edema (naïve: 0.0 ± 0.0) | 2.0 ±0.0 | 2.0 ±0.8 | 2.2 ±0.4 | 1.6 ±0.5 |
| Pulmonary edema (naïve: 0.7 ± 0.5) | 0.0 ±0.0 | 0.7 ±0.8 | 0.3 ±0.5 | 0.2 ±0.4 |
| Alveolar integrity (naïve: 0.0 ± 0.0) | 1.3 ±1.0 | 1.6 ±0.8 | 1.8 ±0.4 | 0.8 ±0.4 |
| Total Lung Injury Score (naïve: 1.0 ± 0.9) | 6.6 ±1.0 | 7.3 ±2.1 | 8.2 ±0.8 | 5.4 ±2.2b |
Figure 3.
Clonidine significantly decreased inflammatory cell infiltration and improved overall lung tissue repair seven days following lung contusion, hemorrhagic shock, and chronic stress (A: without clonidine, B: with clonidine).
The Effects of Clonidine on Lung HMGB-1 and TNF-alpha
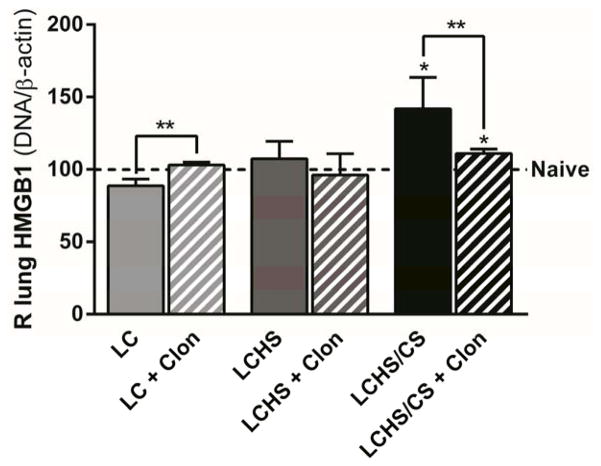
After making the observation that clonidine decreased lung inflammation, we measured lung HMGB1 and TNF-alpha, which are associated with prolonged inflammation and progression of acute lung injury.19, 20 Following acute injury, LC alone and LCHS alone, there was no change in lung HMGB1 expression when compared to naïve (Figure 4). Following injury and chronic stress, lung HMGB1 expression was significantly increased in LCHS/CS group (42% increase, p = 0.010; Figure 4). The administration of clonidine following LCHS/CS significantly decreased lung HMGB1 expression when compared to LCHS/CS (22% decrease, p = 0.031; Figure 4).
Figure 4.
Clonidine decreased HMGB1 expression following LCHS/CS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
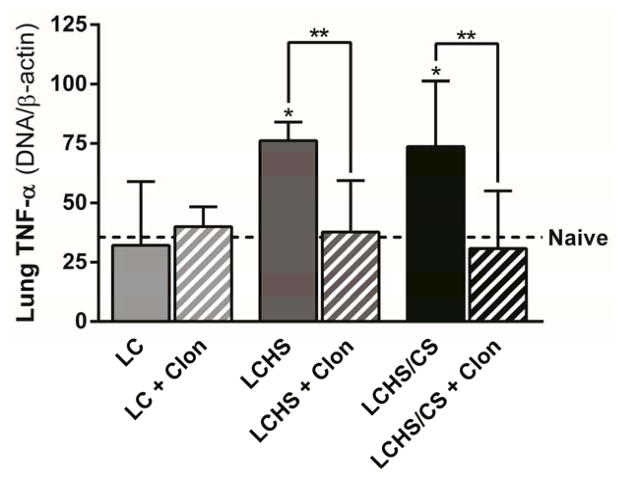
Following LC alone and LC + clonidine there was no change in lung TNF-alpha expression (Figure 5). In the groups with moderate LIS scores on day seven, lung TNF-alpha expression was significantly increased following LCHS (114% increase, p < 0.001; Figure 5) and LCHS/CS (107% increase, p = 0.003). The administration of clonidine following LCHS and LCHS/CS significantly decreased lung TNF-alpha expression in both groups (LCHS + clonidine: 51% decrease, p = 0.016; LCHS/CS + clonidine: 62% decrease, p = 0.031; Figure 5).
Figure 5.
Clonidine decreased TNF-alpha expression following LCHS and LCHS/CS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
Propranolol
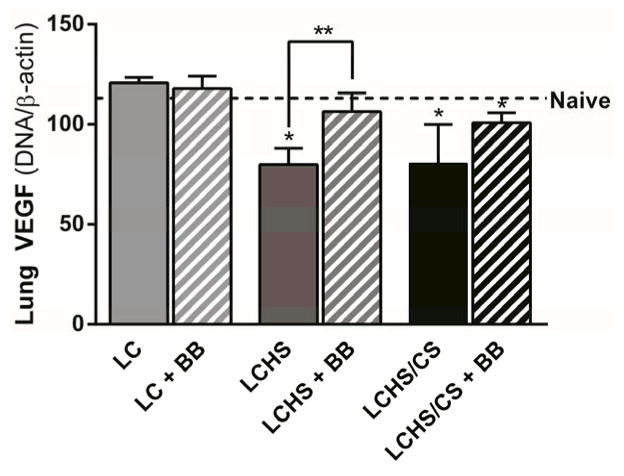
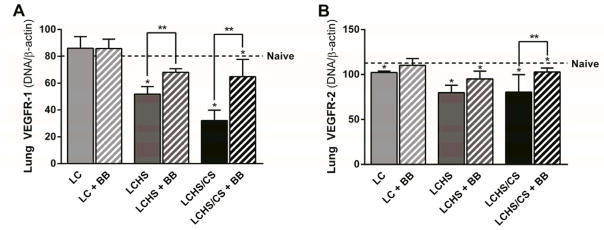
Administration of propranolol following LCHS did significantly increased lung VEGF expression (33% increase, p = 0.006), but not LCHS/CS (25% increase, p = 0.090) (Figure 6). The administration of propranolol did increase VEGFR-1 expression following LCHS (31% increase, p = 0.034), and LCHS/CS (102% increase, p = 0.033), though VEGFR-1 expression remained significantly lower than naïve levels (Figure 7A). Propranolol increased VEGFR-2 following LC, LCHS, and LCHS/CS, but the difference was only statistically significant following LCHS/CS (47% increase, p = 0.004) (Figure 7B). Propranolol did not significantly affect any of the individual LIS components or the total LIS (LCHS + propranolol: 7.6 ±1.5, p = 0.257 vs. naive; LCHS/CS + propranolol: 9.0 ±0.0, p = 0.189 vs. naive).
Figure 6.
Propranolol increased lung VEGF expression seven days following LCHS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
Figure 7.
Figure 7A. Propranolol increased lung VEGFR-1 expression seven days following LCHS and LCHS/CS. 7B: Clonidine increased lung VEGFR-2 expression seven days following LCHS/CS (LC: lung contusion, HS: hemorrhagic shock, CS: chronic stress, Clon: clonidine, *p < 0.05 vs. naive, **p < 0.05 within the group).
Discussion
Following lung contusion alone, injured lungs have healed by day seven and as expected this study did not demonstrate significant changes in lung VEGF or VEGF receptor expression. Our data did indicate that both clonidine and propranolol impact lung VEGF and VEGF receptor expression following lung contusion/hemorrhagic shock and chronic restraint stress. Daily administration of clonidine more consistently and more significantly increased VEGF and VEGF receptor expression, and also decreased HMGB-1and TNF-alpha expression, decreased lung inflammation, and improved lung tissue repair. Lung TNF-alpha expression on day seven correlated poorly with histologic evidence of lung injury, suggesting that other local factors may play more important roles in restoring homeostasis following lung contusion. Differences between the efficacy of clonidine and propranolol in affecting VEGF and VEGF-receptor physiology and lung tissue repair may be related to differences in their mechanisms of action. Although both medications act on stress response pathways, clonidine acts centrally by blocking sympathetic outflow whereas propranolol functions peripherally at the level of the beta-adrenergic receptor.5, 7, 9, 10, 12 Therefore, the superior performance of clonidine is consistent with the hypothesis that the adverse effects of stress-mediated inflammation on wound healing and tissue repair are not limited to beta-adrenergic receptor modulation and downstream pathways like MAPK, JNK, and NF-κB.
In previous studies, persistently low levels of alveolar neutrophil VEGF (6.3 pg/mL) have been associated with prolonged illness in human subjects with Acute Respiratory Distress Syndrome (ARDS), whereas increased levels (13.0 pg/mL) correlated with recovery four days after onset of symptoms.4 Conversely, overexpression of lung VEGF has been associated with pulmonary edema in an animal model utilizing intratracheal delivery of VEGF complementary protein by an adenovirus vector.21 Therefore, too much or too little VEGF may be detrimental to tissue repair. In our model, the addition of HS and CS to LC appear to be associated with a pathologic decrease in VEGF and VEGF receptor expression, and clonidine restores expression levels and improves lung healing.
Previous work has shown that lung VEGF and VEGF receptors may be modulated by potentiating nitric oxide or by inhibiting nitric oxide synthase in the settings of acute and chronic hypoxia.22 In addition, exogenous administration of a pannexin-1 blocker, carbenoxolone, has been shown to decrease LPS-induced HMGB1 release and attenuate LPS-induced acute lung injury.23, 24 However, modulation of VEGF, VEGF receptors, and HMGB1 to promote tissue repair following traumatic lung injury have not been previously reported. The authors surmise that clonidine did not improve lung tissue repair following LCHS because VEGF receptor 1 and 2 expression were not significantly increased by the addition of clonidine following LCHS. This observation supports the hypothesis that both VEGF and VEGF receptor expression play important roles in lung tissue repair following severe trauma.
The major limitations of this study are the lack of mechanistic detail and causal relationships. Also, it may be difficult to translate these findings to the clinical setting, as clonidine may propagate hypotension and has effects lasting for 6–10 hours, such that patient selection may be challenging. Although previous work suggests that direct tissue injury is responsible for changes in VEGF and VEGF receptor expression, closer investigation of stress response pathways upstream of the beta-adrenergic receptor may clarify the mechanism by which clonidine increased VEGF and VEGF receptor expression and improved lung tissue repair following LCHS/CS. Previous work suggests that clonidine may inhibit mast cell degranulation and neutrophil chemotaxis.25 Also, hemorrhagic shock was not performed as an isolated physiologic insult, though it would be interesting to assess the effects of hemorrhagic shock alone on VEGF and VEGF receptor expression following hemorrhagic shock alone. This scenario may apply to trauma patients with major vascular injuries due to penetrating trauma. In addition, these experiments were performed using whole lung samples rather than broncholalveolar lavage fluid specimens, and assessment of lavage fluid would contribute to our understanding of the pathophysiology described in this study. Finally, hematopoietic progenitor cell mobilization and T regulatory cell function appear to play a role in lung tissue repair,14, 15, 26 and the addition of mesenchymal stem cell administration could provide useful insights.
Conclusions
Clonidine increased lung VEGF and VEGF receptor expression following severe injury and chronic stress, decreased inflammatory cell infiltration, and improved tissue repair. Propranolol was less effective in restoring VEGF and VEGF receptor expression levels and did not improve lung tissue repair, suggesting that the stress response pathways responsible for wound healing dysfunction following severe injury may occur upstream of the beta-adrenergic receptor. Future studies should seek to establish causality of VEGF physiology in lung tissue healing and clarify the mechanism by which clonidine increases VEGF and VEGF receptor expression.
Summary.
We hypothesized that clonidine and propranolol would increase VEGF and VEGF-receptor expression and promote lung healing in Sprague-Dawley rats subjected severe trauma and chronic stress. Clonidine increased VEGF and VEGF-receptor expression, decreased HMGB1 expression, decreased lung inflammation, and improved lung tissue repair. Propranolol did not significantly affect VEGF or lung healing.
Acknowledgments
This work was supported in part by grants R01 GM113945-01 (PAE), R01 GM105893-01A1 (AMM), and P50 GM111152–01 (FAM, PAE, AMM) awarded by the National Institute of General Medical Sciences (NIGMS). TJL was supported by a postgraduate training grant (T32 GM-08721) in burns, trauma and perioperative injury by NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003 Jun;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002 Jul;8(7):702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 3.Loftus T, Thomson A, Kannan K, et al. Effects of trauma, hemorrhagic shock, and chronic stress on lung VEGF. J Surg Res. 2016 doi: 10.1016/j.jss.2016.10.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care Med. 2002 Nov 15;166(10):1332–7. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 5.Ballard-Croft C, Maass DL, Sikes P, et al. Activation of stress-responsive pathways by the sympathetic nervous system in burn trauma. Shock. 2002 Jul;18(1):38–45. doi: 10.1097/00024382-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ali A, Herndon DN, Mamachen A, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care. 2015;19:217. doi: 10.1186/s13054-015-0913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001 Oct 25;345(17):1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 8.Horton JW. A model of myocardial inflammation and dysfunction in burn complicated by sepsis. Shock. 2007 Sep;28(3):326–33. doi: 10.1097/01.shk.0000238064.54332.c8. [DOI] [PubMed] [Google Scholar]
- 9.Hofer S, Steppan J, Wagner T, et al. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13(1):R11. doi: 10.1186/cc7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aantaa R, Jalonen J. Perioperative use of alpha2-adrenoceptor agonists and the cardiac patient. Eur J Anaesthesiol. 2006 May;23(5):361–72. doi: 10.1017/S0265021506000378. [DOI] [PubMed] [Google Scholar]
- 11.Bylund DB, Eikenberg DC, Hieble JP, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994 Jun;46(2):121–36. [PubMed] [Google Scholar]
- 12.Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth. 2002 Jan;88(1):101–23. doi: 10.1093/bja/88.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Bible LE, Pasupuleti LV, Gore AV, et al. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015 Sep;158(3):595–601. doi: 10.1016/j.surg.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gore AV, Bible LE, Livingston DH, et al. Can mesenchymal stem cells reverse chronic stress-induced impairment of lung healing following traumatic injury? J Trauma Acute Care Surg. 2015 Apr;78(4):767–72. doi: 10.1097/TA.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S, Ulm J, Sifri ZC, et al. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009 Aug;67(2):315–21. doi: 10.1097/TA.0b013e3181a5c9c7. discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 16.Alamo IG, Kannan KB, Ramos H, et al. Clonidine reduces norepinephrine and improves bone marrow function in a rodent model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2016 Oct 11; doi: 10.1016/j.surg.2016.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce delayed inflammation and pulmonary dysfunction in mice. J Surg Res. 2000 Aug;92(2):206–13. doi: 10.1006/jsre.2000.5899. [DOI] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011 May;44(5):725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutz W, Stetkiewicz J. High mobility group box 1 protein as a late-acting mediator of acute lung inflammation. Int J Occup Med Environ Health. 2004;17(2):245–54. [PubMed] [Google Scholar]
- 20.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997 Apr;155(4):1187–205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 21.Kaner RJ, Ladetto JV, Singh R, et al. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol. 2000 Jun;22(6):657–64. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 22.Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995 Apr;95(4):1798–807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012 Aug 30;488(7413):670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki S, Matsuda Y, Sugawara T, et al. Effects of carbenoxolone on alveolar fluid clearance and lung inflammation in the rat. Crit Care Med. 2004 Sep;32(9):1910–5. doi: 10.1097/01.ccm.0000139621.74965.fb. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CD, Lindgren BR, Andersson RG. Effects of clonidine on the dermal inflammatory cell response of experimental toxic and allergic contact reactions and intradermal hypersensitivity. Int Arch Allergy Appl Immunol. 1987;83(4):371–6. doi: 10.1159/000234371. [DOI] [PubMed] [Google Scholar]
- 26.Badami CD, Livingston DH, Sifri ZC, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007 Sep;63(3):596–600. doi: 10.1097/TA.0b013e318142d231. discussion -2. [DOI] [PubMed] [Google Scholar]