Abstract
Purpose
Seasonal variations in asthma exacerbation (AE) are associated with respiratory virus outbreaks and the return of children to school after vacation. This study aims to elucidate the period, phase, and amplitude of seasonal cycles of AE in 5 different age groups with regard to rhino- and influenza virus epidemics in Korea.
Methods
The number of daily emergency department (ED) visits for AE in all age groups of Korea and the nationwide weekly incidence of rhino- and influenza virus, were obtained for 2008-2012. Fourier regression was used to model rhythmicity, and the Cosinor method was used to determine the amplitude and phase of the cycles in each age group. The cross-correlation function (CCF) between AE and the rhino- and influenza virus epidemics was also calculated.
Results
There were 157,559 events of AE (0.62 events/1,000 individuals/year) during the study period. There were spring and fall peaks of AE in children and adults, but only 1 winter peak in the elderly. The amplitude of the AE peak in infants was higher in spring than in fall (9.16 vs 3.04, P<0.010), and the fall peak was approximately 1 month later in infants than in school children (October 11 vs November 13, P<0.010). The association between AE and rhinovirus was greatest in school children (rho=0.331), and the association between AE and influenza virus was greatest in those aged ≥60 years (rho=0.682).
Conclusions
The rhythmicity, amplitude, and phase of the annual cycle of AE differed among different age groups. The patterns of AE were related to the annual rhino- and influenza virus epidemics.
Keywords: Asthma, seasons, periodicity, rhinovirus, influenza
INTRODUCTION
Asthma exacerbation (AE) can lead to progressive loss of lung function, increase risk of asthma mortality in all age groups,1 and is associated with increased healthcare costs and morbidity.2 Emergency department (ED) visits3,4 and outpatient visits5 for AE in children have seasonal variations. There is a “September peak” in the Northern Hemisphere and a “February peak” in the Southern Hemisphere.3,5 These peaks are associated with the return to school after vacation,3 and coincide with rhinovirus epidemics.3,6 Previous investigations have demonstrated age-related2 and regional5,7 variations of the AE cycle, although differences in the amplitude, phase, and period of this cycle, and its correlation with different age and regional groups, remain to be established. Rhinovirus infection, the most common presentation of asthma in early life,2 is an important trigger of AE in children,8 and its severity is greater in children than in other age groups.9 Influenza virus infection is also a trigger of AE in adults.10 The pattern of the AE cycle varies among age groups, as does the seasonality of rhino- and influenza virus epidemics. However, the association between the seasonal cycle of AE in young and elderly individuals and the rhino- and influenza virus epidemics remain unclear.
The primary objective of our study was to investigate the period, phase, and amplitude of the AE cycle in infants (0-1 year), preschool children (2-5 years), school children (6-17 years), adults (18-59 years), and the elderly (>60 years). We analyzed the cycle of AE in Korean children in light of 2 environmental characteristics unique to Korea: 1) shorter summer vacation (mid-July to mid-August) and longer winter break (January to February), and 2) high humidity during the Korean summer. We also compared the AE cycles of 7 metropolitan areas in Korea using a government database. Our second major objective was to identify the relationship between AE and nationwide rhino- and influenza virus epidemics in each age group.
MATERIALS AND METHODS
Study population
We conducted a retrospective population-based cohort study using the Health Insurance Review and Assessment Service (HIRA) database. Korea has a national healthcare system that covers all physician and hospital services. These data consist of the complete electronic medical records of approximately 98% of the Korean population, and include primary, secondary, and tertiary care.11 We obtained the claims data of the number of ED visits for AE (International Classification of Disease [ICD], 10th version, coded as J45-J46) from January 1, 2008 to December 31, 2012 for patients of all ages. The information extracted included age, sex, area code, date of ED visit, and results of treatment.
Variable description
Individuals with AEs were identified as those who visited an ED for this condition during the study period. This definition of AE, using ICD code data, has 36.4% sensitivity and 94.4% specificity in children aged 2-17 years.12 Subjects were classified into 5 age groups (<2 years, 3-5 years, 6-19 years, 20-59 years, and >60 years). To determine the incidence rate of AE for the different age groups, we used census data obtained from the website of the Ministry of the Interior (rcps.egov.go.kr:8081). This population distribution was retrieved from data from December 31, 2012, which are categorized by age and location. Severe AE is defined by hospitalization, transfer, or death, and other visits as minor exacerbations. The cities classified as metropolitan are Seoul (latitude: 37.57), Busan (latitude: 35.18), Daegu (latitude: 35.87), Gwangju (latitude: 35.16), Incheon (latitude: 37.46), Ulsan (latitude: 35.54), and Daejeon (latitude: 36.35).
Respiratory virus epidemic
Rhino and influenza virus results were obtained from the Korea Centers for Disease Control and Prevention (KCDC), along with weekly virus epidemic data. Briefly, respiratory specimens (throat nasal swab) were collected from patients with acute respiratory infections (ARIs) from 91 primary hospitals nationwide, including pediatric, otolaryngology, and internal medicine facilities. Virus testing was performed using conventional reverse transcription (RT)-polymerase chain reaction (PCR) from 2008 to 2010, and real-time RT-PCR from 2011 to 2012. These data were also used to compare different age groups, because they included patient age, sex, and residential region. We used the rhino- and influenza virus PCR results, and integrated them into the AE database for analysis.
Statistical analysis
The crude number of visits for AE per 1,000 people in this study was determined for individuals of different ages, sexes, and residential regions, and calculated using the census population. Census data in Korea (December 31, 2012) were obtained from the website of the Ministry of the Interior (rcps.egov.go.kr:8081).
The statistical analysis employed Fourier regression to determine the period of seasonal cycles in 5 age groups. Previous studies have used models of this form in numerous applications to describe periodic cycles. Briefly, the data for each age group was fitted to the first 8 consecutive sinusoidal functions by Fourier regression. This can be represented as follows13:
where Y(t) is the number of patients at day t, a0 is the Midline Estimating Statistic Of Rhythm (MESOR), ai and bi are the i-th amplitude and acrophase, ω(=) is related to the shortest period (T), and ε(t) is the error term. Fourier regression produces ω(=), and thus shortest period (T). Using Fourier regression, we searched the count and month of the peak in the seasonal pattern of each age group.
We used the Cosinor regression method to determine the amplitudes and phases of data collected at different times. This method is particularly suitable for the detection of rhythmicity in cross-sectional studies, and for comparison of parameters in different cycles.14 We analyzed 4 parameters—MESOR, amplitude, period, and phase—to characterize seasonal rhythmicity:
| Y(t)=M+Acos(2πt/τ+ϕ)+e(t) |
where M is the MESOR, A is the amplitude, τ is the period, ϕ is the acrophase, and e(t) is the error term.13 Two peaks during the same year had differences in amplitude and phase. We compared the amplitudes of series with 2 peaks, such as spring and fall. The 5-year of data were divided into 2 parts: March 1 to August 31 (spring) and September 1 to February 28 (fall). Applying the Cosinor method with the 2 divided subsets of data allowed us to obtain the amplitude, phase, and MESOR of each part. Phase (the date of a peak) was compared for groups with different ages and of residential regions.
Calculation of the moving average for each week was utilized to determine the number of visits for each age group. To analyze the correlations of the different age groups (infant, preschool, school, adults, and elderly) with regional ED visits among 7 metropolitan areas, we used the cross-correlation function (CCF). When analyzing the CCF between virus infections and AE events of each age group, a week was considered a unit, because data on respiratory virus infection was reported on a weekly basis.
RESULTS
Study subjects
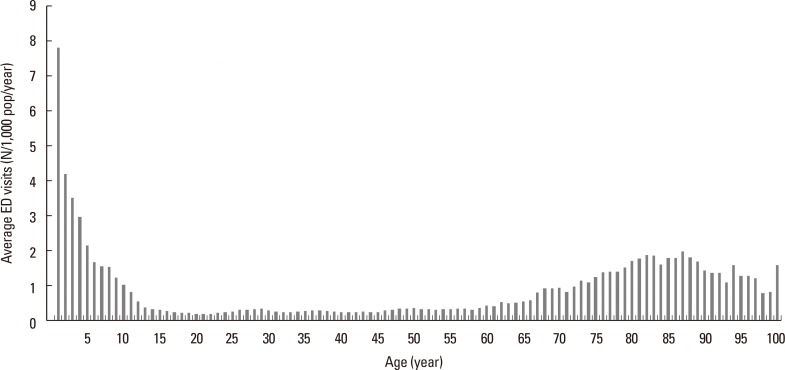
There were 157,559 ED visits (daily mean: 86.2±40.2) for AE in Korea between January 1, 2008 and December 31, 2012, corresponding to a crude ED visitation rate of 0.62 events/1,000 people/year. Overall, the rate was less than 0.1 events/1,000 people/year (n=101) in aged 0-11 months, and highest in those aged 12-23 months (7.8 events/1,000 people/year, n=18,419). The rate quickly declined until it reached 0.4 events/1,000 people/year (n=1,150) in those 13 years. After age 13, the rate did not change until age 61. In the 13 to 61 years' age group, the average ED visit was 0.27±0.06 events/1,000 people/year. The rate again started to increase, which became 0.5 events/1,000 people/year in aged 62 years, and 1.24±0.44 events/1,000 people/year in those aged ≥62 years (Fig. 1). The age-related number of emergency room visits was similar to the age-related prevalence of asthma reported previously.15
Fig. 1. Yearly average ED visits for AE per 1,000 people in different ages during the 5-year study period (January 1, 2008 to December 31, 2012). ED, emergency department; AE, asthma exacerbation.
The male-to-female ratios in the incidence of AE varied among the 5 age groups. There were more males than females in the 3 groups of children aged <18 years, but more females than males for 18- to 59-years and the oldest age group (Table 1). Among the 157,559 ED visits, 18,250 (11.6%) occurred in subjects aged <2 years, 29,716 (18.9%) in those 2-5 years, 24,399 (15.5%) in those 6-17 years-old, 43,515 (27.6%) by those 18-59 years-old, and 41,679 (26.5%) in those aged >60 years.
Table 1. Demographic characteristics of the population of Korea in 2012 and of Koreans admitted to an emergency department (ED) for asthma exacerbation (AE) from 2008 to 2012.
| Variable | Population | ED visits for AE | Crude ED visits for AE per 1,000 people/year | |||
|---|---|---|---|---|---|---|
| N | Male (%) | N | Male (%) | N | Male/Female | |
| Total* | 50,948,272 | 25,504,060 (50.1) | 157,559 | 86,059 (54.6) | 0.62 | 0.68/0.56 |
| Age and Sex | ||||||
| <2 years | 936,649 | 481,116 (51.4) | 18,250 | 11,503 (63.0) | 3.90 | 4.78/2.96 |
| 2-5 years | 1,879,454 | 967,866 (51.5) | 29,716 | 18,671 (62.8) | 3.16 | 3.86/2.50 |
| 6-17 years | 6,875,773 | 3,590,465 (52.2) | 24,399 | 16,223 (66.5) | 0.71 | 0.90/0.50 |
| 18-59 years | 32,847,733 | 16,809,254 (51.2) | 43,515 | 20,858 (47.9) | 0.27 | 0.25/0.28 |
| >60 years | 8,408,663 | 3,655,359 (43.5) | 41,679 | 18,804 (45.1) | 0.99 | 1.03/0.96 |
| Area | ||||||
| Metropolitan** | 23,231,368 | 11,570,589 (49.8) | 65,780 | 35,298 (53.6) | 0.57 | 0.61/0.52 |
| Other *** | 27,716,904 | 13,933,471 (50.3) | 91,779 | 50,761 (55.3) | 0.66 | 0.73/0.60 |
*Population as of December 31st, 2012; **Metropolitan includes Seoul, Busan, Ulsan, Incheon, Daejeun, Gwangju, and Daegu; ***Other includes suburban and rural areas.
Periodicity of the AE cycle in different age groups
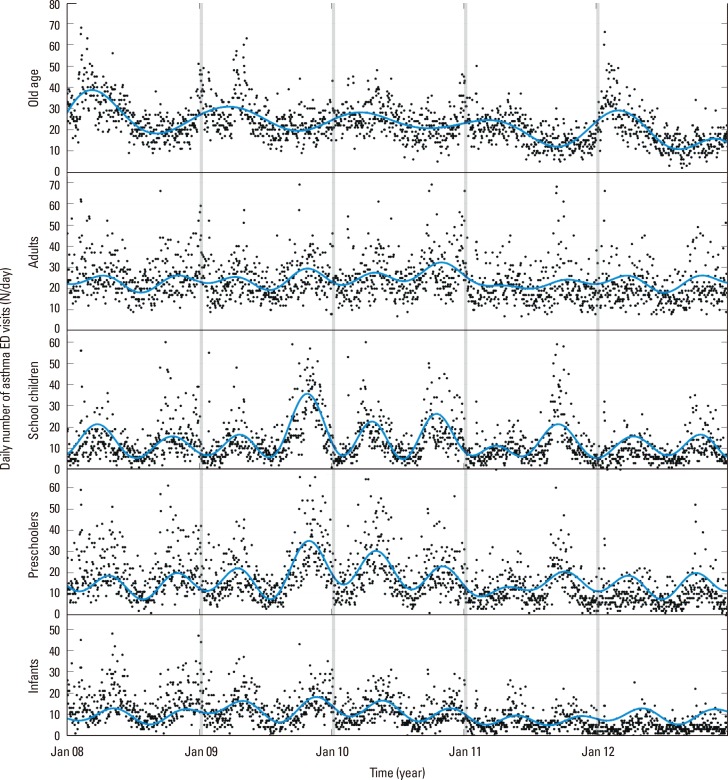
Fig. 2 shows the number of daily ED visits for AE in Korea over 5 years (January 2008 to December 2012) for the 5 age groups. The results of Fourier regression analysis (solid lines) help visualize the patterns of AE cycles in each age group. Notably, there was only 1 maximum per year in subjects aged >60 years (T=365.3 days; which means length of each sinusoidal cycle, 95% CI, 357.0-374.0; P<0.010), but 2 maxima per year in the other 4 age groups (infants: T=182.4 days; 95% CI, 180.9-184.0; P<0.010; preschool children: T=179.2 days; 95% CI, 178.0-180.4; P<0.010; school children: T=181.8 days; 95% CI, 180.5-183.1; P<0.010; and adults: T=180.8 days; 95% CI, 178.4-183.2; P<0.010).
Fig. 2. Daily number of ED visits for AE in each age group during the 5-year study period (0-2 years, infants; 2-5 years, preschool children; 6-17 years, school children; 18-59 years, adults; >60 years, elderly). Blue solid lines indicate fits to Fourier regression (first 8 consecutive sinusoidal functions), and indicate 1 cycle per year in the old age group, and 2 cycles per year in the other groups. ED, emergency department; AE, asthma exacerbation.
Amplitude and concordance of the AE cycle in different age groups
We confirmed that the proportion of ED visits for AE was highest in September for subjects aged 6-17 years (3,959/24,399, 16.2%), and in October (11.8%) for those aged 2-5 years. The maximum was larger in February for those aged >60 years (10.7%), and in May for infants (12.7%). The amplitude of the AE cycle, defined as the difference between the minimum and maximum AE rates, was higher in fall than spring for subjects aged ≥2 years (all age groups: P<0.001) (Table 2). For subjects aged <2 years, the spring amplitude was greater than the fall amplitude (9.16, 95% CI, 8.75-9.75 vs 3.04; 95% CI, 2.46-3.62; P<0.010) (Table 2). In addition, the fall amplitude was greater in those aged 6-17 years (9.09, 95% CI, 7.73-10.44; P<0.010) than in those 18-59 years (4.07, 95% CI, 3.08-5.06; P<0.010) and in preschoolers (6.73, 95% CI, 5.77-7.70; P<0.010).
Table 2. MESOR (midline estimating statistic of rhythm) and amplitude of different age groups derived from Cosinor regression analysis of ED admissions for AE. Each cell shows mean (95% CI).
| MESOR | Amplitude | |||||
|---|---|---|---|---|---|---|
| Spring | Fall | P value | Spring | Fall | P value | |
| <2 years | 10.8 (10.4–11.3) | 9.2 (8.8–9.6) | <0.01 | 9.2 (8.8–9.6) | 3.0 (2.5–3.6) | <0.01 |
| 2-5 years | 15.9 (15.3–16.5) | 16.6 (16.0–17.3) | 0.02 | 3.9 (3.0–4.8) | 6.7 (5.8–7.7) | <0.01 |
| 6–17 years | 10.8 (10.4–11.3) | 15.9 (14.9–16.8) | <0.01 | 3.5 (2.8–4.2) | 9.1 (7.7–10.4) | <0.01 |
| 18–59 years | 23.8 (23.2–24.4) | 23.9 (23.2–24.6) | 0.76 | 1.1 (0.3–2.0) | 4.1 (3.1–5.1) | <0.01 |
AE cycles and peaks showed similar patterns throughout all <60 years age groups (Fig. 2). In Table 3, we calculated cross-correlation coefficients between age groups. Using the school aged group as an indicator, the cross-correlation coefficient was highest for preschoolers (rho=0.794; P<0.010), followed by adults (rho=0.692; P<0.010), and infants (rho=0.518; P<0.01). The similarity was not significant in the elderly (rho=0.115; P=0.140) (Table 3).
Table 3. Cross correlation coefficients and lag times* for AE in different pairs of age groups.
| <2 years | 2-5 years | 6-17 years | 18-59 years | >60 years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rho | Lag | rho | Lag | rho | Lag | rho | Lag | rho | Lag | |
| <2 years | 1 | 0 | 0.785 | −1 | 0.518 | −2 | 0.600 | 0 | 0.525 | 0 |
| 2–5 years | 0.794 | 0 | 0.694 | 0 | 0.402 | 1 | ||||
| 6–17 years | 0.692 | 1 | 0.115 | 1 | ||||||
| 18–59 years | 0.512 | 0 | ||||||||
| >60 years | 1 | 0 | ||||||||
*Lag time indicates the order of AE cycles in each age group. For example “-1” means an AE peak one week earlier, and “+1” means an AE peak one week later.
Phase and lag time of the AE cycle in different age groups
While the eldest group only showed single peak per year, all the other groups showed dual peaks, which can be divided into spring and fall cycles. Those 4 groups had different phases of the AE cycle. The Cosinor regression model of those 4 groups results phase value, which means the date of the highest prevalence. The phases of the 4 groups were statistically different from each other (Table 4). The spring and fall AE peaks were earliest for subjects aged 6-17 years, followed by preschoolers, adults, and infants for the spring peaks, and followed by adults, preschoolers, and infants for the fall peaks (Table 4). More specifically, the AE peak for school children preceded those of preschool children by 10 days and infants by almost 1 month.
Table 4. Phase* of the AE cycle in 4 age groups based on Cosinor regression analysis.
| Age group | Spring | Fall | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | Actual day (95% CI) | P value | Estimate (95% CI) | Actual day (95% CI) | P value | |
| <2 years | −1.44 (−3.28 to −2.88) | 18-May (26-Mar to 7-Apr) | 0.02 | −1.62 (−1.81 to −1.43) | 13-Nov (7-Nov to 18-Nov) | <0.01 |
| 2–5 years | −2.26 (−2.49 to −2.02) | 24-Apr (18-Apr to 1-May) | <0.01 | −2.46 (−2.60 to −2.31) | 20-Oct (16-Oct to 24-Oct) | <0.01 |
| 6–17 years | −2.61 (−2.80 to −2.42) | 14-Apr (9-Apr to 20-Apr) | Ref | −2.75 (−2.90 to −2.60) | 11-Oct (7-Oct to 16-Oct) | Ref |
| 18–59 years | −1.79 (−2.55 to −1.03) | 8-May (16-Apr to 30-May) | <0.01 | −2.70 (−2.94 to −2.45) | 13-Oct (6-Oct to 20-Oct) | 0.62 |
*Phase indicates the peak time of AE before the reference date. The maximum is -2π, which means -182.5 day before January 1 (Fall) or July 1 (Spring). Therefore, for the youngest age group (<2 years), a phase of -1.44 during spring means -41.8 days before July 1 and a phase of -1.62 during fall means -47.1 days before January 1.
Incidence and peak of AE in different geographic areas
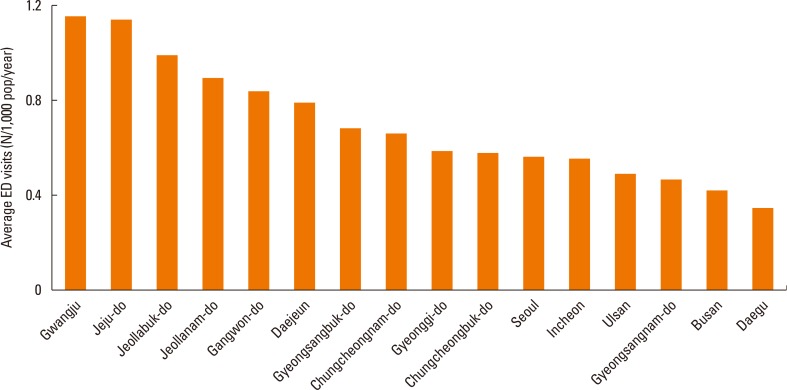
The rate of ED visits for AE was highest in Gwangju (1.16/1,000/year), followed by Jeju-do (1.15/1,000/year), Jeollabuk-do (0.99/1,000/year), and Jeollanam-do (0.90/1,000/year) (Fig. 3). Busan and Daegu had the lowest rates (0.41 and 0.34/1,000/year). Comparing the rate of ED visits for AE showed that some regions (i.e., Gwangju) had nearly 3-times higher ED visit rates than others regions (i.e., Daegu). To determine whether this phenomenon is related to the age structure of the populations in the different regions, we compared ED visits for AE of all 7 metropolitan cities in each age group. The results showed that the ED visits for AE was always 3-5-fold higher in metropolitan areas (data not shown).
Fig. 3. Yearly average ED visits for AE per 1,000 people during the study period in 7 metropolitan areas and 9 other areas during the 5-year study period. Gwangju had 3-times more daily visits than Daegu. ED, emergency department; AE, asthma exacerbation.
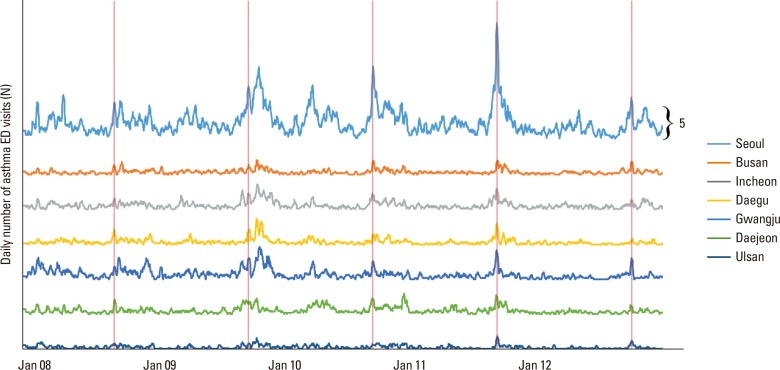
To compare the time of the AE peak with region, we compared peak dates of those aged 6-17 years. The result indicated that an approximately one week later peak occurred in coastal cities of lower latitude, such as Busan and Ulsan. The cross-correlation coefficient between locations ranged from 0.423 to 0.724 (Fig. 4, Supplementary Table).
Fig. 4. Moving average of daily number ED visits for AE in 7 metropolitan areas during the 5-year study period. During each year, the first AE outbreak (fall, red vertical lines), occurred almost concurrently in all 7 cities. ED, emergency department; AE, asthma exacerbation.
Cross-correlation between AE cycle and rhino- and influenza virus epidemics in different age groups
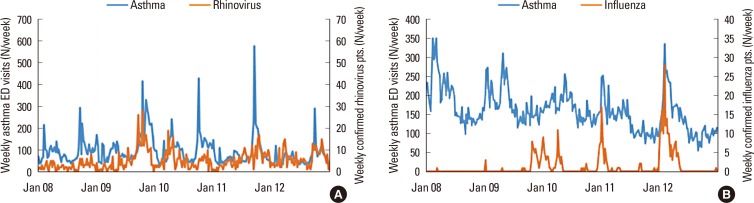
There were 57,228 viral PCR samples available, with 22.4% being positive for influenza virus and 14.9% positive for rhinovirus. The cross-correlation between AE and rhinovirus prevalence was highest in school children (rho=0.331; P<0.010), but there were no statistically significant correlations for preschoolers and infants (Fig. 5A). The cross-correlation coefficient between AE and influenza virus prevalence was higher for the elderly (rho=0.682; P<0.010) than the other age groups (rho=0.458-0.545; P<0.010) (Fig. 5B).
Fig. 5. Weekly ED visits for AE, rhinovirus infection, and influenza virus infection over 5 years. (A) Number of ED visits for AE and incidence of rhinovirus infection in school-age children. Cross-correlation analysis indicated a significant relationship (rho=0.331; P<0.010). (B) Number of ED visits for AE and incidence of influenza virus infection in the elderly. Cross-correlation analysis indicated a significant relationship (rho=0.682; P<0.010). ED, emergency department; AE, asthma exacerbation.
DISCUSSION
This large population-based cohort study investigated the effect of residential region and patient age on the annual AE cycle, and the association of the AE cycle with rhino- and influenza virus epidemics in Korea. Our findings are comparable to previous studies that demonstrated a September peak of ED visits for AE in school children, with later peaks for preschoolers, infants, and adults. We also identified 2 cycles of AE per year in children and adults, but only 1 cycle per year in the elderly. The amplitude of the AE cycle was highest in the spring for infants, suggesting that cause of AE could vary among the age groups. It is noteworthy that the prevalence of ED visits for AE was 3- to -5 fold higher in the geographic region where AE was most common than in the other regions. Further investigations are needed to explain these geographical differences. The peak of the AE cycle in coastal cities of lower latitude appeared 1 week after that of other cities. The rhinovirus epidemic cycle was synchronized with the AE cycle in school children, whereas the influenza epidemic cycle was synchronized with the AE cycle in the elderly.
The present study confirms that AE in school children (age 6-17) has an especially high prevalence in fall,3,16,17,18 correlates with the rhinovirus epidemic,6 and occurs earlier in this age group than in the other age groups in Korea.4 In contrast, the AE peak in infants (ages <2) occurs more frequently in the spring. The pattern of AE in preschool children (ages 2-5) was intermediate, in terms of amplitude and phase, between those of infants and school children. Previous research also indicated that AE is common in spring, though not as remarkable as in fall,3,16,19 and is more evident in younger children.19,20 These studies are consistent with our results.
Tree pollens are less likely to be the major cause of AE, because school children are more prone to sensitization by pollens than preschoolers or infants.17,19 Although rhinovirus is ubiquitous and can cause infections year-round,21 the incidence rate of rhinovirus infection is highest in spring and fall.8 This suggests that rhinovirus is a major cause of AE in spring. Previous research indicates that 30% of the cases of AE can be explained by rhinovirus,6,8 but its effect differs according to age group.5 This implies an additive or synergistic interaction between the rhinovirus epidemic and other risk factors,8,17 such as other respiratory viruses,19 pollutants,22 inhaled allergens,23 or lack of asthma control.5,8 In Korea, high dust levels during spring have significant effects on respiratory diseases in children.24 In addition, during the Asian dust season, concurrent epidemics of human metapneumovirus, parainfluenza virus, adenovirus, and/or bocavirus20,25 could affect the amplitude of the AE cycle in infants and preschool children. Further research is needed to identify factors that differentiate spring AE from fall AE in children.
We observed a single annual peak of AE in the elderly.2,4 This pattern is not merely a delayed form of that observed in the younger groups, but a unique pattern.3 This implies the existence of factors other than those responsible for the spring AE,2 such as indoor pollutants, indoor allergens, cold and dry air conditions, tobacco smoke, and epidemics of other viruses.4,5 AE in the elderly had a higher correlation with influenza virus infection than in other age groups, suggesting that the viruses that trigger AE differ according to age groups.
We divided the country into 7 metropolitan cities and 9 provinces to study AE in different geographic regions, and then examined spatial distribution data of the September peak in school children. We found that for all age groups, the prevalence of AE rate differed by up to 3-fold among regions. This contrasts with previous research3 that found AE is significantly more prevalent in densely populated and urban areas7 or different cultural segments.5 We believe that the regional differences in AE reported in the current study may not be adequately explained by differences in weather conditions or pollutants. Further research on asthma prophylaxis and use of asthma control medications5 may help explain these geographic variations.
The 2 main strengths of our research are that we examined the entire population of Korea and that analyzed PCR results for rhinovirus and influenza virus. The government database covers all ED visits at all levels of the healthcare system for acute AE. This enabled us to separately analyze different age groups. Previous studies focused on the correlation of AE with allergens and weather in limited populations, but our study examined all residents of Korea, regardless of age and region of residence. Furthermore, our use of PCR results allowed us to track the rhino- and influenza virus epidemics during the 5-year study period. The present research is of importance in that it suggested a prototype of seasonal AE cycle using statistical analytics tools, in conjunction with Cosinor regression and Fourier regression.
A limitation of our study is that we did not have patient-level data to evaluate the clinical impact of AE.17 Such data is critical for differentiating asthma from acute bronchiolitis in children, and asthma from chronic obstructive pulmonary disease (COPD) in the elderly.18 This lack of patient-level data may have affected our identification of factors responsible for AE. Second, we did not have data on potential AE triggers, such as pollutants, allergens, stress, or other virus epidemics.4,26 In addition, we included subjects with ICD codes for AE, but since it is difficult to diagnose asthma and/or AE in children, particularly in infants aged <2 years, there is a possibility that we did not include some patients with asthma and/or AE in this age group. Finally, we did not include data from children with mild asthma (not requiring emergency treatment).
This study reports important new information on the annual AE cycle. We used analytical statistics to identify the period, amplitude, and phase of age- and location-specific AE cycles. We found that AE in school children (ages 6-17) peaks in fall, and correlates with the rhinovirus epidemic. In younger children, AE was more prominent in spring, implying a lower correlation with rhinovirus and the presence of other underlying causes. In contrast, the elderly had a single peak in winter, suggesting a relationship with the influenza virus epidemic. An intriguing finding is that the AE prevalence differed by region within Korea, even though culture, weather, and pollution levels had little variation. Further research is required to identify the factors responsible for these regional differences in AE.
ACKNOWLEDGMENTS
This work was partially supported by Korea Centers for Disease Control and Prevention (KCDC) Influenza and Respiratory Viruses Surveillance Project. We also would like to thank the Health Insurance Review and Assessment Service (HIRA) for providing data.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIAL
Cross correlation coefficients and lag times* for different pairs of locations
References
- 1.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 2.Szefler SJ, Chmiel JF, Fitzpatrick AM, Giacoia G, Green TP, Jackson DJ, et al. Reply: to PMID 24290281. J Allergy Clin Immunol. 2014;133:1776–1777. doi: 10.1016/j.jaci.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Larsen K, Zhu J, Feldman LY, Simatovic J, Dell S, Gershon AS, et al. The annual September peak in asthma exacerbation rates. Still a reality? Ann Am Thorac Soc. 2016;13:231–239. doi: 10.1513/AnnalsATS.201508-545OC. [DOI] [PubMed] [Google Scholar]
- 4.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–728. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen HA, Blau H, Hoshen M, Batat E, Balicer RD. Seasonality of asthma: a retrospective population study. Pediatrics. 2014;133:e923–e932. doi: 10.1542/peds.2013-2022. [DOI] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laor A, Cohen L, Danon YL. Effects of time, sex, ethnic origin, and area of residence on prevalence of asthma in Israeli adolescents. BMJ. 1993;307:841–844. doi: 10.1136/bmj.307.6908.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song DJ. Rhinovirus and childhood asthma: an update. Korean J Pediatr. 2016;59:432–439. doi: 10.3345/kjp.2016.59.11.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toivonen L, Schuez-Havupalo L, Karppinen S, Teros-Jaakkola T, Rulli M, Mertsola J, et al. Rhinovirus infections in the first 2 years of life. Pediatrics. 2016;138:e20161309. doi: 10.1542/peds.2016-1309. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders DL, Gregg W, Aronsky D. Identifying asthma exacerbations in a pediatric emergency department: a feasibility study. Int J Med Inform. 2007;76:557–564. doi: 10.1016/j.ijmedinf.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HS, Choi GS, Cho JS, Kim YY. Epidemiology and current status of allergic rhinitis, asthma, and associated allergic diseases in Korea: ARIA Asia-Pacific workshop report. Asian Pac J Allergy Immunol. 2009;27:167–171. [PubMed] [Google Scholar]
- 16.Won YK, Hwang TH, Roh EJ, Chung EH. Seasonal patterns of asthma in children and adolescents presenting at emergency departments in Korea. Allergy Asthma Immunol Res. 2016;8:223–229. doi: 10.4168/aair.2016.8.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135:1465–1473.e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggo RM, Scott JG, Galvani AP, Meyers LA. Respiratory virus transmission dynamics determine timing of asthma exacerbation peaks: evidence from a population-level model. Proc Natl Acad Sci U S A. 2016;113:2194–2199. doi: 10.1073/pnas.1518677113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harju T, Keistinen T, Tuuponen T, Kivelä SL. Seasonal variation in childhood asthma hospitalisations in Finland, 1972-1992. Eur J Pediatr. 1997;156:436–439. doi: 10.1007/s004310050632. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Choi JY, Kim NY, Kim JW, Baek JH, Baek HS, et al. Clinical risk factors associated with the development of wheezing in children less than 2 years of age who required hospitalization for viral lower respiratory tract infections. Korean J Pediatr. 2015;58:245–250. doi: 10.3345/kjp.2015.58.7.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J. 2015;45:774–789. doi: 10.1183/09031936.00062714. [DOI] [PubMed] [Google Scholar]
- 22.Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10:e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keegan AD, Shirey KA, Bagdure D, Blanco J, Viscardi RM, Vogel SN. Enhanced allergic responsiveness after early childhood infection with respiratory viruses: are long-lived alternatively activated macrophages the missing link? Pathog Dis. 2016;74:ftw047. doi: 10.1093/femspd/ftw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Hashizume M, Ueda K, Shimizu A, Takeuchi A, Kubo T, et al. Asian dust and pediatric emergency department visits due to bronchial asthma and respiratory diseases in Nagasaki, Japan. J Epidemiol. 2016;26:593–601. doi: 10.2188/jea.JE20150309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SW, Cho E, Koh HY, Shin J, Baek JH, Shin YH, et al. Effect of solar irradiation on serum specific immunoglobulin E to house-dust mite. Allergy Asthma Proc. 2015;36:44–50. doi: 10.2500/aap.2015.36.3845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cross correlation coefficients and lag times* for different pairs of locations