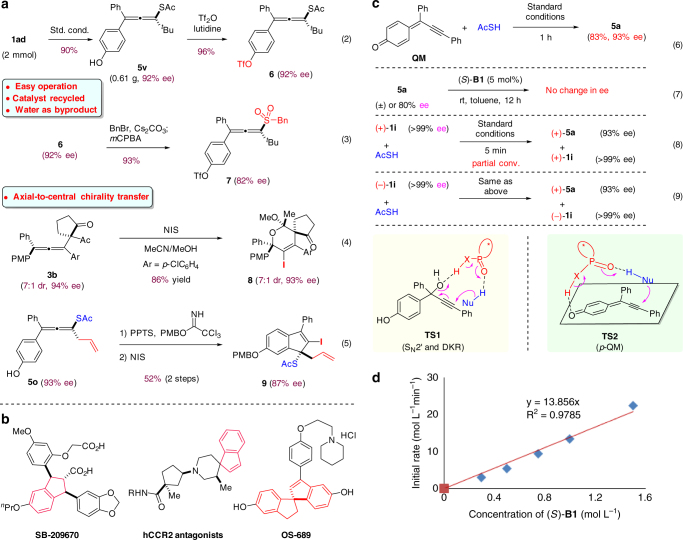
Fig. 6.
Representative product transformations and mechanistic studies. a Triflation of the free hydroxy group (Eq. 2), transformation of the thioacetate moiety to a sulfone unit (Eq. 3), and NIS-mediated cyclizations of the chiral tetrasubstituted allenes proceed via the axial-to-central chirality transfer (Eqs. 4–5). b Important molecules bearing chiral indene or indane units. c Control experiments and possible mechanism. The reactions suggest that the 1,8-addition to p-QM is rate-determining (Eq. 6) and the conjugate addition step is irreversible (Eq. 7). The product stereochemistry was purely determined by the chiral catalyst, regardless of the absolute configuration of substrate (Eqs. 8–9), which rules out the possible SN2’ pathway (via TS1). d Kinetic study indicated that the reaction is first-order in catalyst, suggesting that one catalyst molecule is involved in the rate-determining transition state