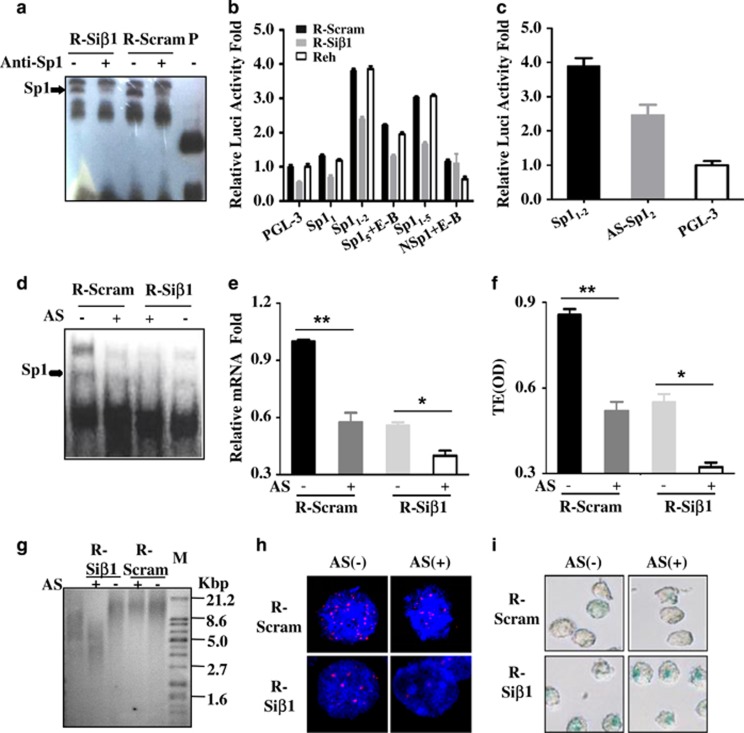
Figure 3.
Loss of β-Arrestin1 reduces the transcription of hTERT. (a) Different Reh cells were collected. We performed supershift by EMSA on purified nucleus protein to detect Sp1 binding with the hTERT promoter. (b) Reh cells were transfected by different plasmids with various cloned fragments. The luciferase activity was detected by dual-luciferase reporter assay and normalized by Renilla luciferase. The relative luciferase activity was viewed as 1.0 in Reh with a PGL-3-basic vector. (c) The anti-sense oligonucleotide was designed for the binding sites (−28 to −36 bp) of the hTERT promoter, measuring the relative luciferase activity, and comparing between Reh with or without the anti-sense oligonucleotide. R-Siβ1 cells and R-Scram cells were transfected by anti-sense oligonucleotide of Sp12 (−28 to −36 bp). The cells' total DNA and protein were collected. Then we detected the binding of the hTERT promoter with Sp1 by EMSA (d) measured the relative hTERT mRNA fold by RT-PCR (e) detected the activity of telomerase by PCR-ELISA (f) measured the length of telomere by Southern blot (g) and FISH (h), respectively, and obtained representative images through SA-β-gal staining (i). Anti-Sp1, antibody for Sp1; AS, anti-sense oligonucleotide. AS, anti-sense oligonucleotide of Sp12 (−28 to −36 bp); Ctrl, Reh cells not transfected with anti-sense oligonucleotide of Sp12 (−28 to −36 bp); E-B, E-box region; NSP, the fragment excluding the binding sites for Sp1; P-C, positive control; TE, the activity of telomerase; TRF, the average length of telomere. Scale bar, 10 μm