Figure 2. MUC16 expression increases EGFR expression and stability.
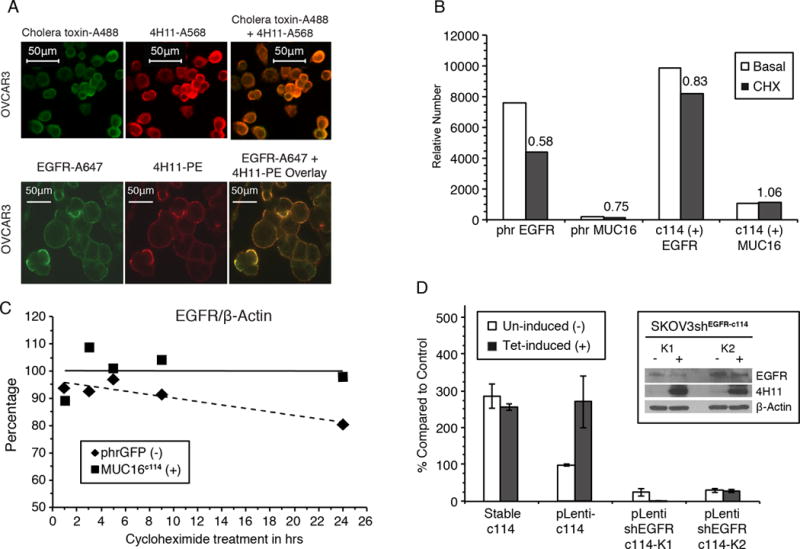
A) Cell surface MUC16 is localized in lipid rafts and co-localizes with EGFR. Cholera toxin localizes to lipid rafts on the cell surface (green label) (Alexa488) and co-localizes with the red-labeled anti-MUC16 (Alexa568) on the cell surface of OVCAR 3 cell line. In the same cell line, EGFR (Alexa647 green label) also co-localizes with MUC16 (Phycoerythrin).
B) MUC16 increased EGFR expression. Cells were labeled wth anti-EGFR-A647 and relative number estimated by FACS geometric mean fluorescence. Relative cell surface EGFR expression was reduced to 58% of untreated levels upon 24 h of CHX exposure in SKOV3-phrGFP. In contrast, in the SKOV3-MUC16c114 cells, there was an increase in EGFR geometric mean fluorescence, which decreased to 83% of that of the control after CHX exposure. MUC16c114 mean fluorescence is not reduced by CHX.
C) Expression of MUC16c114 stabilizes total EGFR after cycloheximide. SKOV3-cells with and without MUC16 expression were exposed to cycloheximide and expression of EGFR species were compared at various times. Densitometry of the EGFR/β-actin ratio illustrates that there is a steady loss of EGFR over time in SKOV3-phrGFP cells during CHX exposure. In contrast, the total level of EGFR in SKOV3-MUC16c114 cells is maintained, showing EGFR stabilization compared to the MUC16-negative control cell line.
D) MUC16c114 enhancement ofmatrigel invasion is dependent on EGFR. Tetracycline induction of SKOV3-MUC16c114(tet) cells resulted in an invasive phenotype similar to the stable SKOV3-MUC16c114 (SKOV3c114). When a short hairpin RNA knockdown of EGFR (shEGFR) was introduced into SKOV3-MUC16c114(tet) cells, tetracycline induced expression of MUC16 (4H11 positive protein in insert) but did not increase matrigel invasion (n=3).
