Figure 3. Protein-protein interactions of MUC16c114 with EGFR and Integrin β1 require Galectin-3.
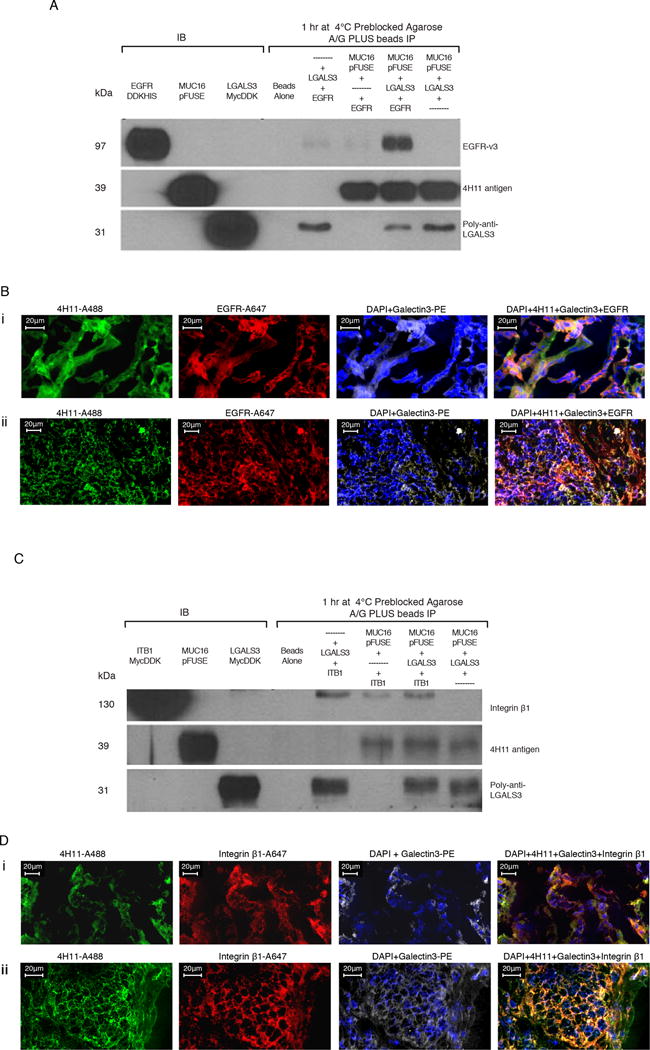
A) Immunoprecipitation (IP) of EGFR, MUC16c57-114-pFUSE, and Galectin-3. Triple immunoprecipitation studies were performed as described in the methods. In control lanes (1-3), each single protein is present by immunoblot. In the immunopreciptation lanes(5-8), the anti-MUC16 4H11antibody binds to theMUC16c57-114-pFUSE and is precipitated bound to the A/G beads. LGALS3 is present in the lane positive for MUC16c57-114-pFUSE, but not EGFR alone. EGFR is detected only when both LGALS3 and MUC16c57-114-pFUSE are present.
B) MUC16, Galectin-3, and EGFR protein co-localization in ovarian cancer explants and human ovarian cancer sections. Immunofluorescence co-localizationof a section from an ovarian cancer explant with EGFR-A647 (red), Galectin-3-PE (white) and 4H11-PE (green) for MUC16, or a combination of all three with DAPI (blue) in the last panel is performed as described in the methods. The lower panels show similar co-localization studies in a snap frozen tumor section from a patient with ovarian cancer.
C) Interaction between MUC16 and Integrin β1 requires Galectin-3. Triple immunoprecipitation studies were perfomred as in 3A. In the combination lanes (5–8) anti MUC16 antibody 4H11staining shows that MUC16c57-114-pFUSE is consistently bound to the A/G beads. LGALS3 binds in the lane positive for MUC16c57-114-pFUSE, but Integrin β1 is detected only when both LGALS3 and MUC16c57-114-pFUSE are present.
D) MUC16, Galectin-3, and Integrin β1 protein co-localization in ovarian cancer explants and human ovarian cancer sections. Immunofluorescence staining of an ovarian cancer cell explant with Integrin β (red), Galectin-3 (white) and 4H11-PE (green) for MUC16, or a combination of all three with DAPI (blue) in the last panel. The lower panels show similar co-localization studies in a snap frozen tumor section from a patient with ovarian cancer.
