Figure 6. Targeting glycosylation-dependent MUC16 function.
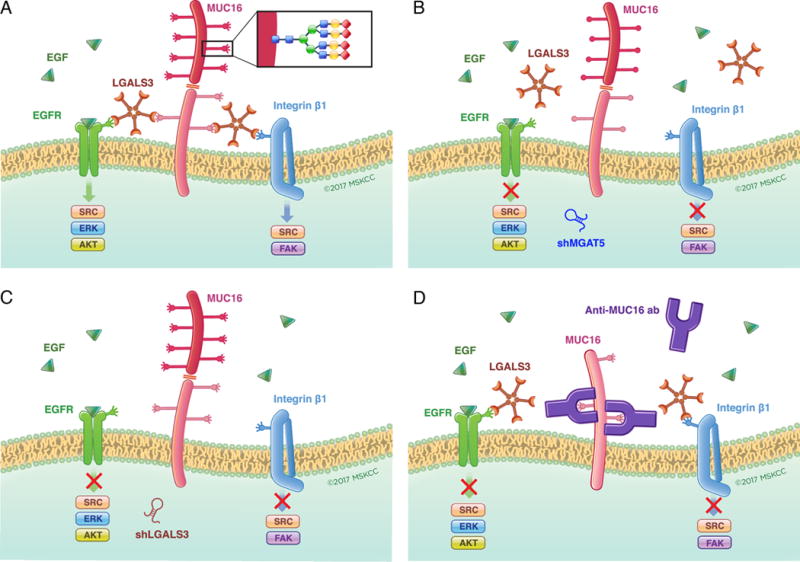
In Panel A, the dependence of MUC16 action on the presence of high complexity N-glycosylation at specific asparagine residues, Galectin-3 pentamers and growth factor receptors is graphically summarized. Panels B and C illustrate how loss of tumor-derived Galectin-3 or the tumor cell glycosylation enzyme MGAT5 may each block this interaction through depletion of a key component of the glycosylation-galectin-growth factor receptor interaction. Panel D illustrates how exogenous anti-MUC16 glycosylation site antibody interferes with galectin binding to MUC16 N-glycosylation sites to block-dependent oncogenic behaviors.
