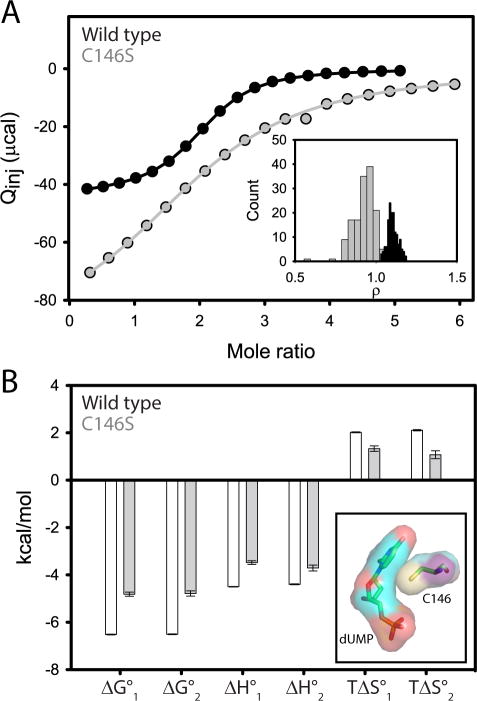
Figure 1.
dUMP binding to wild-type and C146S TSase by ITC at 25 °C. Wild-type data were presented previously 22. (A) ITC isotherms for wild-type (black) and C146S (gray) binding to dUMP with fitted lines shown. Note the dimeric TSase concentrations in the cell were 206 μM and 696 μM for wild-type and C146S TSase respectively. The inset shows the cooperativity factor, ρ, which is the ratio of the intrinsic association constants for binding to the free and singly bound enzymes. (B) ITC-derived thermodynamic parameters for the two dUMP binding events. Note the less favorable ΔG°bind is the net of less favorable enthalpic and entropic components. The inset shows the surfaces of dUMP and C146 from the wild-type dUMP-complex x-ray model (pdbid 1BID).