Abstract
Background:
Anticoagulants have been used in the treatment of several circulatory diseases and thrombotic disorders, and in the blood sampling for hematologic analysis. Sulfated polysaccharides (SP), which have anticoagulant properties, are found in most seaweeds, including Caulerpa spp.
Objective:
The study generally aimed to evaluate the potential anticoagulant property of Caulerpa lentillifera.
Methodology:
The whole plant of fresh C. lentillifera was washed thoroughly with distilled water and manually expressed to obtain the extract. C. lentillifera extract was tested in two phases. Phase one utilized nine male albino rabbits, which were randomly and equally allocated into three groups: (1) negative control (oral distilled water and subsequent in vitro mixing of extracted blood with normal saline solution), (2) positive control (oral aspirin and subsequent in vitro mixing of extracted blood with normal saline solution), and (3) experimental group (oral distilled water and in vitro mixing of extracted blood with C. lentillifera extract). Blood coagulation was evaluated by measuring the clotting time using the slide and tube methods. In phase two, peripheral blood from three apparently healthy adult dogs were used. Blood collection was performed thrice. In each collection, the sample was divided into five aliquots: (1) negative control (normal saline solution), (2) positive control (ethylenediaminetetraacetic acid [EDTA]), and (3-5) experimental treatments at 0.1, 0.15, and 0.2 ml of C. lentillifera extract. Coagulation was evaluated by measuring the clotting time using the tube method.
Results:
Phase one results revealed significant differences on the clotting time between the negative and the positive and experimental groups (P < 0.05), and no significant differences on the clotting time between the positive and the experimental groups (P > 0.05). In phase two, all blood samples mixed with EDTA did not clot, while the negative control had an average clotting time of 2.01 min. Blood mixed with 0.2 ml of C. lentillifera extract had the longest coagulation time (15.49 min). Simple linear regression revealed a positive significant correlation (multiple R = 0.9450, R2 = 0.8931, P = 0.02) implying dose-dependent anticoagulant potential. The study showed that C. lentillifera extract may have a potential anticoagulant property due to its component SP.
Keywords: Anticoagulant, Caulerpa lentillifera, dog, rabbit
Introduction
Homeostasis, the ability of the body to maintain a relatively stable environment, is essential for survival. This includes the normal blood circulation, characterized by the continuous flow of blood to the different organ systems of the body. A substance (heparin) present in the plasma prevents blood from clotting to allow normal circulation.6 This substance is called an anticoagulant.8 Anticoagulants are used in the treatment for several circulatory diseases and thrombotic disorders, including atrial fibrillation, pulmonary embolism, deep vein thrombosis, venous thromboembolism, congestive heart failure, stroke, myocardial infarction, and genetic or acquired hypercoagulability.1 It has also been used for treatment against snake venoms.10
In routine human and animal disease diagnosis, complete blood count (CBC) is usually performed. This procedure necessitates blood extraction and mixing with an anticoagulant, usually ethylenediaminetetraacetic acid (EDTA).17 EDTA, currently considered as an environmental pollutant,11 is usually the preferred chemical substance for blood samples for CBC. EDTA has been shown to cause hemolysis in common carp blood.18
Anticoagulant therapy may be expensive, and alternative cheaper medicines from natural sources may be explored. The search for alternative sources of anticoagulants has risen as a result of the increasing demand for safer anticoagulant clinical therapy.9 While several studies have demonstrated the antithrombin and anticoagulant properties of marine algae Caulerpa spp.,4,14-16 no studies have been conducted investigating Cualerpa lentillifera as a potential anticoagulant. Hence, this study was conducted.
The study generally aimed to evaluate the anticoagulant property potential of C. lentillifera. Specifically, it sought to assess the in vitro anticoagulant effect of C. lentillifera aqueous extract in the blood of male rabbits (Oryctolagus cuniculus) under experimental conditions and in the blood of dogs (Canis familiaris) under field conditions.
Methodology
Research design and subjects
The experimental study on rabbits utilized a completely randomized design. Briefly, 9 male albino rabbits (4-6 months old, 1.0-1.25 kg) were used and randomly divided into 3 groups: Aspirin (positive), negative control (normal saline solution), and experimental extract (C. lentillifera). Individual cage placement of rabbits was also randomly assigned. Rabbits were acclimatized for 1 week before the start of the actual experiment. For the field testing of the in vitro effect of C. lentillifera extract in canine blood, 3 apparently healthy dogs were used. Blood samples were treated with the following concentrations accomplished in triplicate: 0.10 ml of the extract in 0.5 ml whole blood, 0.15 ml of the extract in 0.5 ml whole blood, and 0.20 ml of the extract in 0.5 ml whole blood. The negative control had no additives while the positive control was mixed with EDTA.3 Consent was obtained from the owner of the dogs.
Plant sample collection, identification, and research environment
Plant samples were obtained from a plantation in Brgy. Kalawisan, Lapu-lapu City, Cebu, Philippines. The identity of the samples as C. lentillifera was confirmed by experts from the Bureau of Fisheries and Aquatic Resources (Region VII) and the Biology and Environmental Studies Program of the University of the Philippines Cebu. Crude extract was prepared at the University of Southern Philippines Foundation, while the conduct of the experiment in rabbits and dogs were performed at the Pharmacy Laboratory of the University of Southern Philippines-Foundation, Salinas Drive, Lahug, Cebu City, and at the GPY Veterinare Animale Veterinary Clinic, Tres de Abril, Punta Princesa, Cebu City, respectively.
Preparation of extract and aspirin administration
Fresh samples of the entire plant of C. lentillifera were washed thoroughly with distilled water and allowed to drain. Plant extract was obtained by the manual expression method using a muslin cloth. Extracted juice was placed in an Erlenmeyer flask covered with cork and stored (refrigerated) until further use. On the other hand, locally available aspirin (80 mg/tablet) was used and crushed using mortar and pestle, and was placed in another Erlenmeyer flask and added with commercially prepared normal saline to a final volume of 24 ml to a make an approximately 3 mg/ml concentration. The solution was prepared on the day of the oral administration to the positive control rabbits, which were fasted for 12 h before the procedure.
Blood extraction and tube transfer
Two hours after oral administration of solutions to the rabbits, blood was aseptically extracted from the marginal ear vein. Before extraction, the collection site was disinfected with 70% alcohol. Blood was extracted using a sterile 3 ml syringe with gauge 21 needle. Before transferring to the designated blood tubes, the needle was removed to prevent hemolysis. Blood was gradually transferred to the tubes at 45° angle. The same protocol was followed in the blood extraction in dogs, except that the collection site was the saphenous vein.
Evaluation of anticoagulant potential
For the experiment involving rabbits, an arbitrary dose of 0.5 ml C. lentillifera per 1.5 ml of collected blood was used in the positive (aspirin) and experimental groups. Blood from the negative control used sterile normal saline solution at a similar dose of 0.5 ml per 1.5 ml blood. Two methods were used to evaluate the anticoagulant potential: (1) the test tube method, where the coagulation time of the collected blood (1.5 ml) was recorded after mixing with the test solution (observation time was within 30 min, every 3-5 min), and (2) the slide method, where blood samples were placed in a slide (after mixture with the test solution as required) and observed regularly until clotting or the presence of fibrin is seen. In the latter method, the blunt part of the needle was used to stir blood in the slide and to check for fibrin presence. Before the administration of test solutions, baseline mean clotting times of each treatment group were also obtained. For the canine samples, freshly collected blood samples were evaluated for the presence of clot in a 0.5 ml microtainer every 3-5 min after mixing with the C. lentillifera extract. Before the transfer of the collected blood to the tubes, the extracts were already placed in advance.
Data processing and analysis
Clotting time was manually recorded on a tally sheet and was subsequently transferred to Microsoft Excel. Data were analyzed using one-way ANOVA with subsequent post hoc analyses (Tukey HSD) for comparison between groups. For the canine sample data, simple linear regression was also performed to determine the correlation between average clotting and concentration of extract in the mixture. Results indicating P < 0.05 were considered as significant.
Ethical considerations
The procedures performed in this study were guided by the principles of animal welfare, Animal Welfare Act of the Philippines (RA 8485) and AO 45 of the Bureau of the Animal Industry. The study was also approved by the Institutional Animal Care and Use Committee and Research Office of the University of Southern Philippines-Foundation, Salinas Drive, Lahug, Cebu City, Cebu, Philippines.
Results and Discussion
In phase one, baseline clotting time of the different treatment groups revealed no significant differences regardless of the evaluation method. In the post-treatment, highly significant differences were absorbed in both evaluation methods (P < 0.001). This difference was accounted between the negative and the positive control, and the negative control and the experimental group. There was no significant difference observed between the positive control and experimental group in both evaluation methods (Table 1). Results imply that the C. lentillifera extract was able to prolong the clotting time, with similar results with the positive control (aspirin). Aspirin has properties that can reduce the ability of the blood to clot, and thus, it is often used in the treatment of conditions associated with blood clots, including heart attacks.5
Table 1.
Mean clotting time (seconds) of rabbit (Oryctolagus cuniculus) blood treated with C. lentillifera extract (3 per group; n=9)

In phase two, all blood samples that were mixed with EDTA did not clot, while the negative control had an overall average clotting time of 2.01 min. Blood mixed with a concentration of 0.2 ml of crude extract was found to have the longest coagulation time, followed by 0.15 ml and 0.1 ml (Table 2). Simple linear regression revealed a positive significant correlation (Multiple R = 0.9450, R2 = 0.8931, P = 0.02). On the other hand, analysis on the comparison between concentrations revealed a highly significant difference (P < 0.001, F = 62.33, df = 3). Post hoc analyses further revealed that except between 0.1 ml and 0.15 ml concentration which revealed a significant difference (P < 0.05), high significant differences (P < 0.01) were found between all other concentrations (table not shown).
Table 2.
Mean clotting time (minutes) of canine blood mixed with C. lentillifera extract
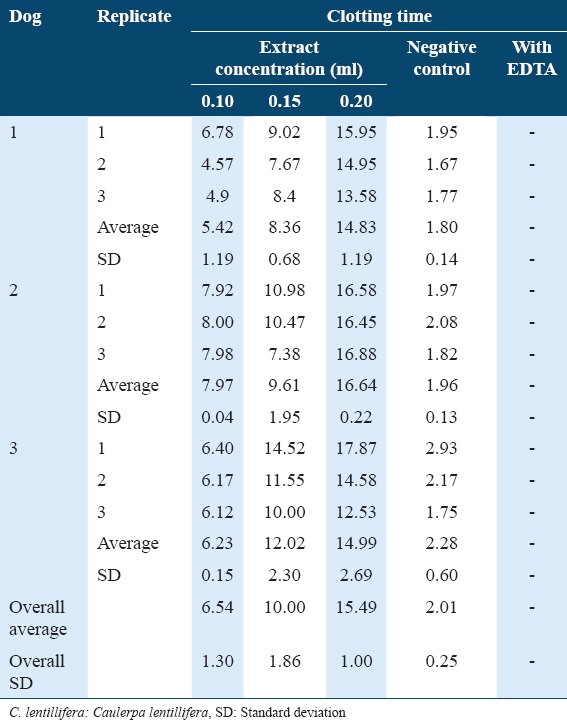
The inability of the blood samples mixed with EDTA to clot indicates that blood sample collection was done appropriately. The overall average clotting time of the negative control shows that the dog sources were within the normal range,2 indicating normal clotting functions. On the other hand, blood mixed with the highest concentration (0.2 ml) had the longest coagulation time (Table 1), which may imply that higher concentrations of the crude extract may lead to longer coagulation time of the blood. This finding is also supported by the simple linear regression result. In a study by Rodrigues et al.,16 the anticoagulant property of a related species Caulerpa cupressoides var. lycopodium was found to be dose-dependent. The aforementioned study also extracted sulfated polysaccharides (SP) from the selected seaweed.
SPs are structural components of the cell wall of marine algae, in which they are found in high concentrations.12-15 The use of these molecules as alternative sources of anticoagulants is justified by the fact that algae are phylogenetically distant from mammals, significantly reducing contamination by viral particles.7
Another study evaluated the in vitro anticoagulant activity of SP fractions from red alga Halymenia pseudofloresia using citrated rabbit plasma and observed marked changes in activated partial thromboplastin time.14 The fractions obtained in the first (464.20, 211.60, 103.50, and 101.70 IU/mg) were more active compared to those from the third extraction (137.10, 96.50, and 89.20 IU/mg). Its actions were considered superior to the existing heparin standard (100 IU/mg) and SPs from the same genus species Halymenia sp.15
Comparing between concentrations, the analysis revealed a highly significant difference (Table 2). Post hoc analyses using Tukey HSD further revealed that except between 0.1 ml and 0.15 ml concentration which revealed a significant difference (P < 0.05), high significant differences (P < 0.01) were found between all other concentrations (table not shown). Results show that the crude extract of C. lentillifera has a potential anticoagulant property. A study by Rodrigues et al.16 isolated an antithrombin-dependent SP from the green alga C. cupressoides that has in vivo anti- and pro-thrombotic effects. It will be interesting to explore similar study to C. lentillifera.
Conclusion
The crude extract of C. lentillifera has a potential in vitro anticoagulant property. The results of the study necessitate further studies. The potential of C. lentillifera as an in vitro anticoagulant for diagnostic purposes must be explored using SP, concentrations or different fractions of the seaweed. Its use as a potential therapy for thrombotic disorders must also be explored in vivo.
References
- 1.Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants:A review of the pharmacology, dosing, and complications. Curr Emerg Hosp Med Rep. 2013;1:83–97. doi: 10.1007/s40138-013-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byars TD, Ling GV, Ferris NA, Keeton KS. Activated coagulation time (ACT) of whole blood in normal dogs. Am J Vet Res. 1976;37:1359–61. [PubMed] [Google Scholar]
- 3.Cerón JJ, Carli E, Tasca S, Martinez-Subiela S, Caldin M. Evaluation of EDTA hematology tubes for collection of blood samples for tests of secondary hemostasis in dogs. Am J Vet Res. 2008;69:1141–7. doi: 10.2460/ajvr.69.9.1141. [DOI] [PubMed] [Google Scholar]
- 4.Costa MS, Costa LS, Cordeiro SL, Almeida-Lima J, Dantas-Santos N, Magalhães KD, et al. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var flabellata . J Appl Phycol. 2012;24:1159–67. [Google Scholar]
- 5.Guirguis-Blake JM, Evans CV, Senger CA, O'Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events:A systematic evidence review for the U.S. preventive services task force. Ann Intern Med. 2016;164:804–13. doi: 10.7326/M15-2113. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE. Guyton and Hall Textbook of Medical Physiology. USA, Philadelphia: Saunders Publishing Company; 2015. [Google Scholar]
- 7.Leite EL, Medeiros MG, Rocha HA, Farias GG, Da Silva LF, Chavante SF, et al. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schröederi . Plant Sci. 1998;132:215–28. [Google Scholar]
- 8.Merriam-Webster.com. anticoagulant. [Last accessed on 2016 Nov 1]. Available from: http://www.merriam-webster.com/dictionary/anticoagulant .
- 9.Das Neves Amorim RC, Rodrigues JA, Holanda ML, De Souza Mourão PA, Benevides NM. Anticoagulant properties of a crude sulfated polysaccharide from the red marine alga Halymenia floresia (Clemente) C. Agardh. Acta Sci Biol Sci. 2011;33:255–61. [Google Scholar]
- 10.Oliveira CZ, Maiorano VA, Marcussi S, Sant'Ana CD, Januário AH, Lourenço MV, et al. Anticoagulant and antifibrinogenolytic properties of the aqueous extract from Bauhinia forficata against snake venoms. J Ethnopharmacol. 2005;98:213–6. doi: 10.1016/j.jep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Oviedo C, Rodríguez J. EDTA:The chelating agent under environmental scrutiny. Quimica Nova. 2003;26:901–5. [Google Scholar]
- 12.Painter TJ. In: The Polysaccharides. Aspinall GO, editor. Vol. 2. New York, NY: Academic Press; 1983. pp. 195–285. [Google Scholar]
- 13.Pereira MG, Benevides NM, Melo MR, Valente AP, Melo FR, Mourão PA. Structure and anticoagulant activity of a sulfated galactan from the red alga Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr Res. 2005;340:2015–23. doi: 10.1016/j.carres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues JA, Torres VM, de Alencar DB, Sampaio AH, Farias WRL. Extraction and anticoagulant activity of sulfated polysaccharides of the red marine algae Halymenia pseudofloresia. Rev Cienc Agron. 2009;40:224–31. [Google Scholar]
- 15.Rodrigues JA, Torres VM, De Alencar DB, Sampaio AH, Farias WR. Natural heparinoids isolated from rutafíceas (Halymenia sp.) from arriving on the coast of cearense. Acta Sci Biol Sci. 2010;32:235–42. [Google Scholar]
- 16.Rodrigues JA, Queiroz IN, Quinderé AL, Vairo BC, Mourão PA, Benevides NM. An antithrombin-dependent sulfated polysaccharide isolated from the green alga Caulerpa cupressoides has in vivo anti-and prothrombotic effects. Cienc Rural. 2011;41:634–9. [Google Scholar]
- 17.Thompson CB, Diaz DD, Quinn PG, Lapins M, Kurtz SR, Valeri CR. The role of anticoagulation in the measurement of platelet volumes. Am J Clin Path. 1983;80:327–32. doi: 10.1093/ajcp/80.3.327. [DOI] [PubMed] [Google Scholar]
- 18.Witeska M, Wargocka W. Disodium EDTA used as anticoagulant causes hemolysis in common carp blood. Turk J Vet Anim Sci. 2011;35:99–104. [Google Scholar]


