Fig. 1.
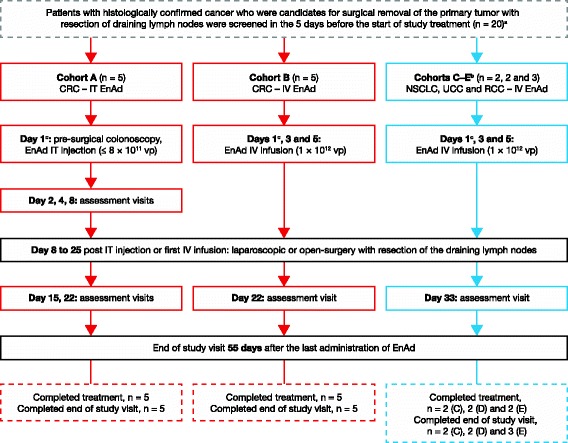
Study design and patient disposition. a Two patients were excluded at screening because of inadequate renal function, and one because of bowel obstruction. b Cohorts C–E initiated after completion of cohort A and B comparison phase. c Enadenotucirev (EnAd) administration days also counted as assessment visits (i.e. cohort A: day 1; cohort B: days 1, 3, and 5)
