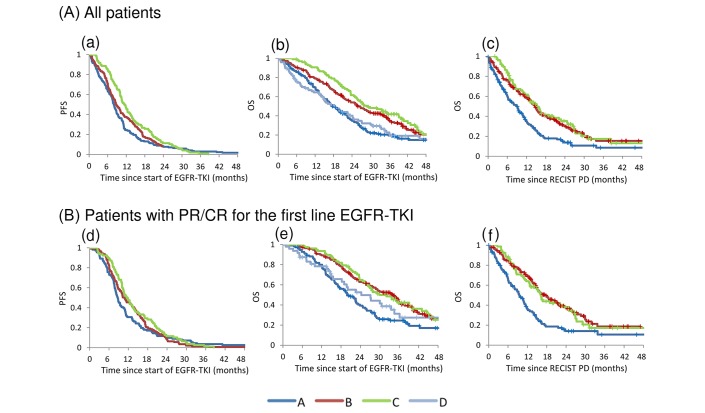
Figure 2.
(A) Survival time. All patients. (a) PFS since the start of EGFR-TKI (groups A, B and C). (b) OS since the start of EGFR-TKI (groups A, B, C and D). (c) OS since RECIST PD diagnosis (groups A, B and C).(B) Survival time. Patients with PR or CR for first-line EGFR-TKI. (d) PFS since the start of EGFR-TKI (groups A, B and C). (e) OS since the start of EGFR-TKI (groups A, B, C and D). (f) OS since the diagnosis of RECIST PD (groups A, B and C). CR, complete response; EGFR, epidermal growth factor receptor; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.