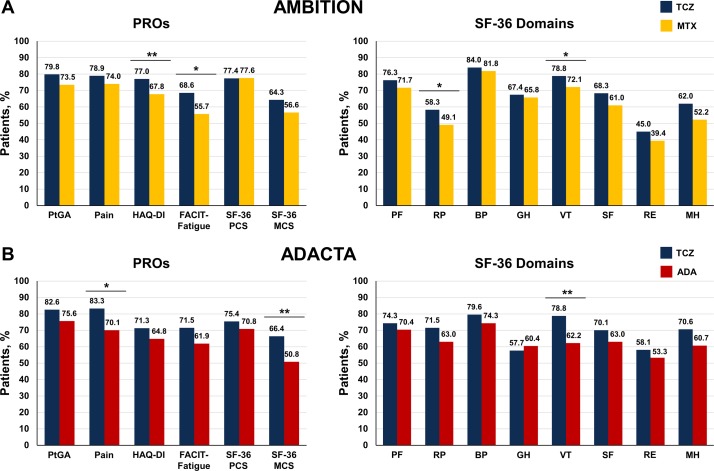
Figure 2.
Proportion of patients reporting improvement ≥MCID at 24 weeks in the (A) AMBITION and (B) ADACTA trial populations. Analyses were performed using the per-protocol population in the AMBITION (TCZ, n=265; MTX, n=259) and the intention-to-treat population in ADACTA (TCZ, n=163; ADA, n=162) and adjusted for site (AMBITION)/region (ADACTA), baseline score (ADACTA) and duration of RA. The MCID for PROs were defined as follows: HAQ-DI: ≥0.22; PtGA: ≥10; patient pain: ≥10; FACIT-Fatigue: ≥4; SF-36 PCS/MCS: ≥2.5; SF-36 domains: ≥5.0. ADA, adalimumab; BP, bodily pain; FACIT, Functional Assessment of Chronic Illness Therapy; GH, general health; HAQ-DI, Health Assessment Questionnaire Disability Index; MCS, mental component summary; MCID, minimum clinically important differences; MH, mental health; MTX, methotrexate; PCS, physical component summary; PF, physical functioning; PROs, patient-reported outcomes; PtGA, patient global assessment; RE, role-emotional; RP, role-physical; SF, social functioning; SF-36, Short Form-36; TCZ, tocilizumab; VT, vitality. *p<0.05; **p<0.01.