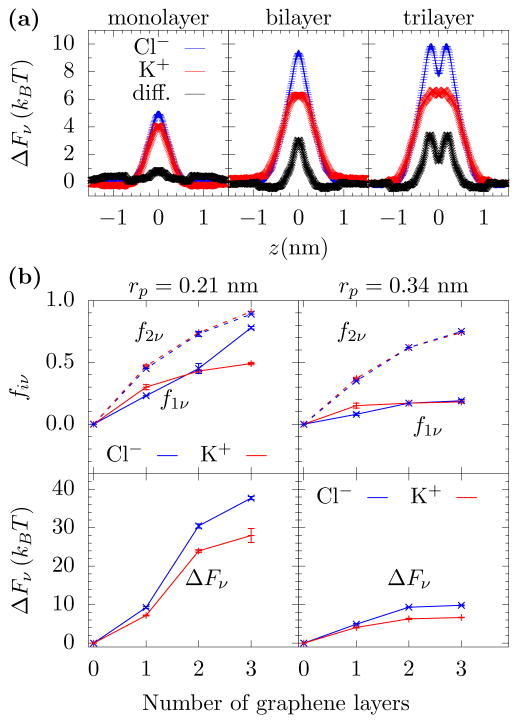
FIG. 2. Free energy barriers and dehydration.
(a) Free energy barrier versus K+ and Cl− location, z, on the pore axis as they cross mono-, bi-, and tri-layer graphene pores with radius 0.34 nm. As the number of layers increases, the energy barrier becomes more substantial and a difference between the two ion types appears. (b) Fractional dehydration in the first and second layer (f1ν and f2ν) for K+ and Cl−, where the ion is at the position of its free energy maximum in the pore. When the pore radius is less than the first hydration layer radius (about 0.3 nm), then both the first and second hydration layers lose a substantial amount of their water molecules (upper left panel). However, with just a slightly larger pore radius, rp = 0.34 nm, the first hydration layer retains most of its water but the second layer still loses a significant number of water molecules (upper right panel). The free energy barriers (lower panels) will increase with the number of graphene layers, as a “short pore” interferes less with the hydration than the longer pores. However, while dehydration is the mechanism by which selectivity occurs, water loss is not the sole predictor of selectivity. As Eq. 1 shows, one also needs the hydration layer energies. The Cl− ion has a larger hydration energy and, thus, even for the same fiν, Cl− will be selected against. Error bars are ±1 standard error from five parallel simulations.