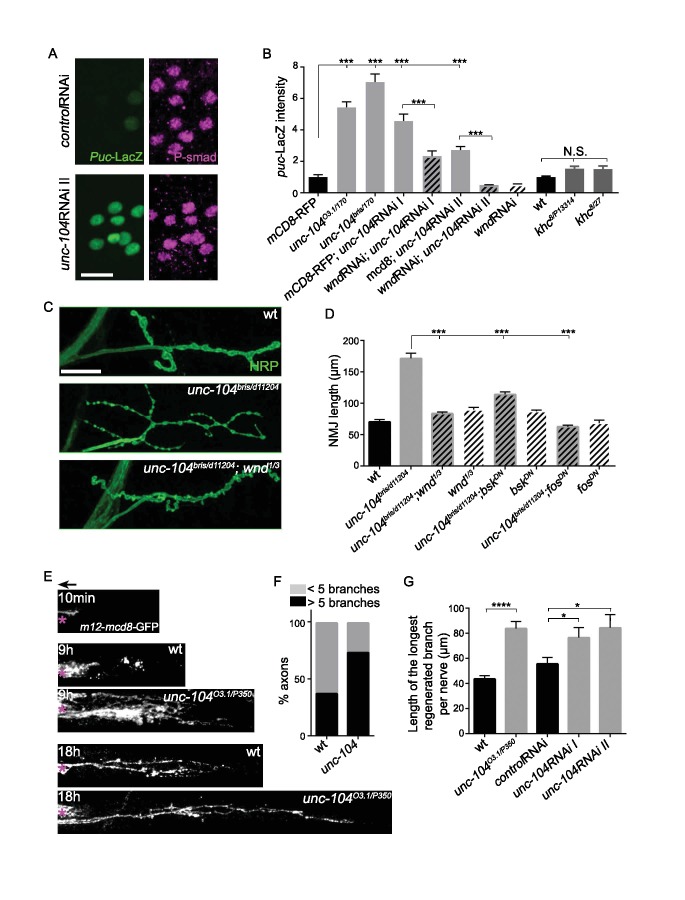
Figure 4. The Wnd signaling pathway is activated in unc-104 mutants.
(A) Images of motoneuron cell bodies near the midline of larval ventral nerve cord, immunostained for motoneuron nuclear marker phospho-SMAD (magenta) and the Wnd/JNK nuclear reporter puc-LacZ (green). UAS-RNAi lines (including UAS-moody RNAi as a control) were driven by pan-neuronal BG380-Gal4. At least 3 dorsal abdominal clusters of motoneurons per VNC from 6 animals per genotype were examined. (B) Quantification of puc-lacZ expression in multiple unc-104 mutant backgrounds. Unc-10403.1 and unc-104bris are hypomorphic alleles, while unc-104170 is a null allele. In addition we tested two independent unc-104 RNAi lines (vdrc 23465, I and TRiP BL43264, II). Unc-104 RNAi II was accompanied with Dicer2 expression to facilitate the knock-down. Co-expression of wnd-RNAi, compared to UAS-mCD8-ChRFP reduced the effect of unc-104 knockdown. The ‘control’ genotype has UAS-mCD8-ChRFP, (which serves as a control for dosage of UAS lines). Quantification methods are described in Experimental Procedures. (C) Presynaptic bouton morphology and branching at the NMJ, viewed via immunostaining for HRP (membrane marker) at muscle 4. The presynaptic arbor was over-branched in unc-104 bris mutants compared to wt (Canton S) control animals, and this was rescued in unc-104bris; wnd1/3 double mutants. (D) Quantification of presynaptic overgrowth was estimated by measuring the total NMJ length (from the most proximal to the most distal bouton of the presynaptic nerve terminal at muscle 4, labeled via anti-HRP staining). The data shown used unc-104bris and wnd1/3 mutations, while UAS-bskDN and UAS-fosDN were driven by elav-Gal4. wt control animals were Canton S. (E) Regenerative axonal sprouting of m12-Gal4, UAS-mcd8-GFP labeled axons 10 min, 9 hr or 18 hr after nerve crush from wt compared to unc-104O3.1(hypomorph)/P350(null() mutant animals. Asterisk (*) indicates the injury site and arrow indicates the direction of the cell body. By 9 hr after nerve crush injury, axons in wild type animal initiate new growth via short filopodia-like branches from the proximal axonal stump. At 18 hr, a few branches are stabilized and grow either towards the distal axon or the cell body. In comparison, unc-104 mutants showed a marked increase in new axonal branches at 9 hr, and at 18 hr these new axonal branches showed similar stabilization but extended nearly twice as far as wild type axons. (F) 9 hr after injury, the percentage of axons which contain more than 5 identifiable branches per axon. (G) The length of the longest branch per nerve at 18 hr after injury. unc-104O3.1/P350 mutant and 2 independent unc-104 RNAi lines and control RNAi (moody-RNAi) (driven by m12-Gal4) were examined. All data are represented as mean ±SEM; N.S., not significant; ****p<0.0001, ***p<0.001,*p<0.05, Tukey test for multiple comparison; Scale bar, 20 μm.