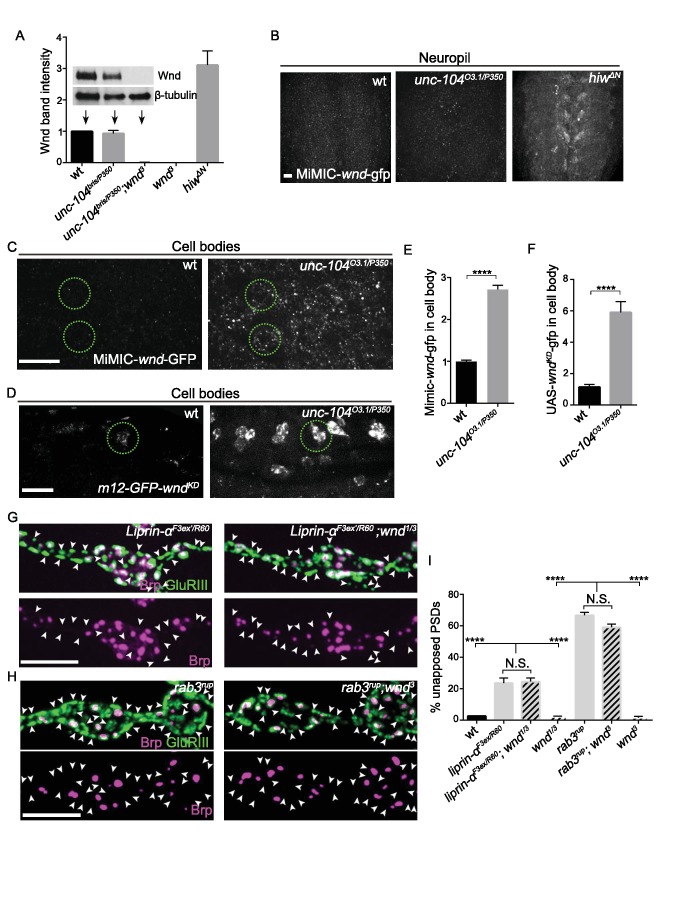
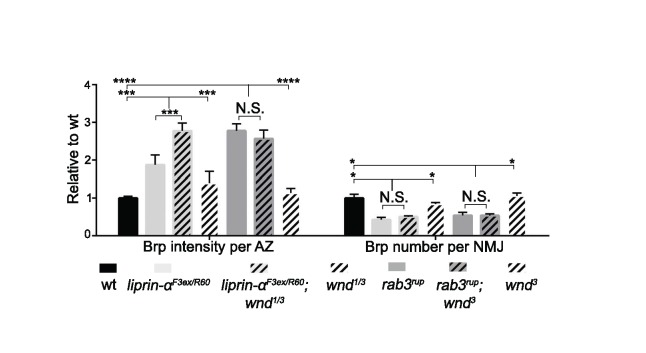
Figure 5. Wnd signaling is activated via an unconventional mechanism in unc-104 mutants.
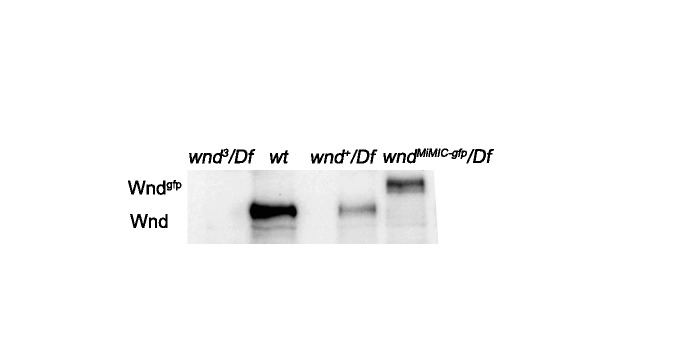
(A) Representative Western blot of larval whole brain extracts for endogenous Wnd and β-tubulin, and quantification of Wnd levels normalized to β-tubulin band intensity (n ≥ 3). Mutants examined include unc-104bris(hypomoprh)/P350(null), wnd3 and hiwΔN. (B) Images of larval ventral nerve cords from MiMIC-wnd-GFP animals immunostained for GFP. Note the increased neuropil signal in hiw mutants, but not control or unc-104 mutants. This also indicates that the MiMIC-wnd-GFP is, like endogenous Wnd (Collins et al., 2006), subject to regulation by Hiw. (C) Images of motoneuron cell bodies in MiMIC-wnd-GFP animals immunostained with antibody against GFP. Compared to (B), these images are collected in a higher magnification and focal plane to view the motoneuron cell bodies. Two representative cell bodies are marked by green circles. At least 6 animals were examined per genotype. (D) Images of SNc motoneuron cell bodies via live-imaging in larvae expressing UAS-GFP-wndkd (which is kinase dead to avoid pathway activation) driven by m12-Gal4. A representative cell body is marked by a green circle. At least 6 animals were examined per genotype. (E–F) Quantification of GFP signal intensity from (C–D) in cell bodies, normalized to wt (control) animals. Note that ectopically expressed GFP-wnd kinase dead protein has a higher basal expression level which allows for increased sensitivity in detecting changes to Wnd protein. (G–H) Representative images of presynaptic Brp and postsynaptic GluRIII from (G) liprin-αF3ex/R60 and liprin-α F3ex/R60;wnd1/3 and (H) rab3rup and rab3rup;wnd3. Unapposed GluRIII-labeled PSDs are highlighted by arrowheads. Wt is Canton S.. (I) Percentage of unapposed GluRIII-labeled PSDs from (G) and (H). All data are represented as mean ±SEM; N.S., not significant, ****p<0.0001; Tukey test for multiple comparison; Scale bar (B–D) 20 μm and (G–H) 5 μm. For additional data, see Figure 5—figure supplements 1–3.