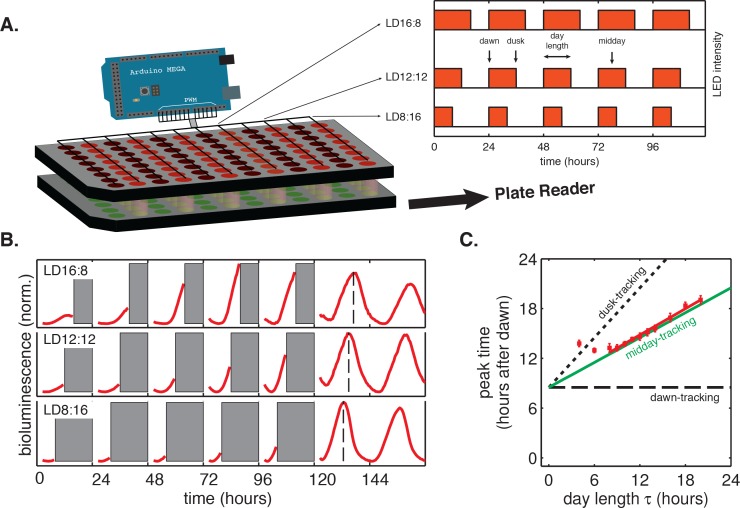
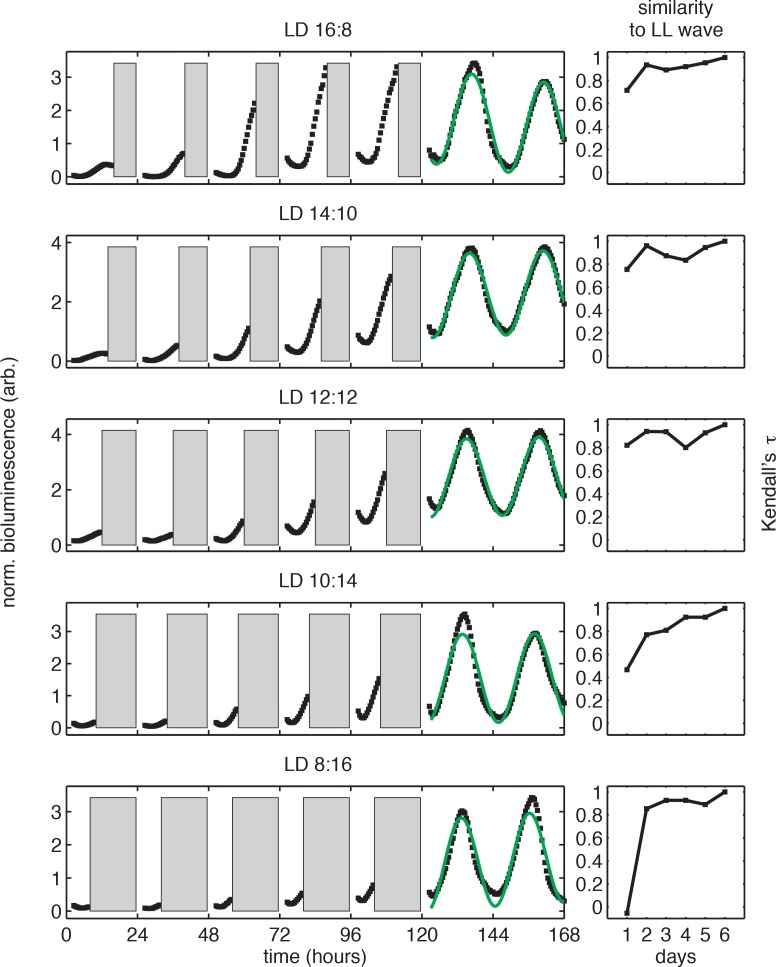
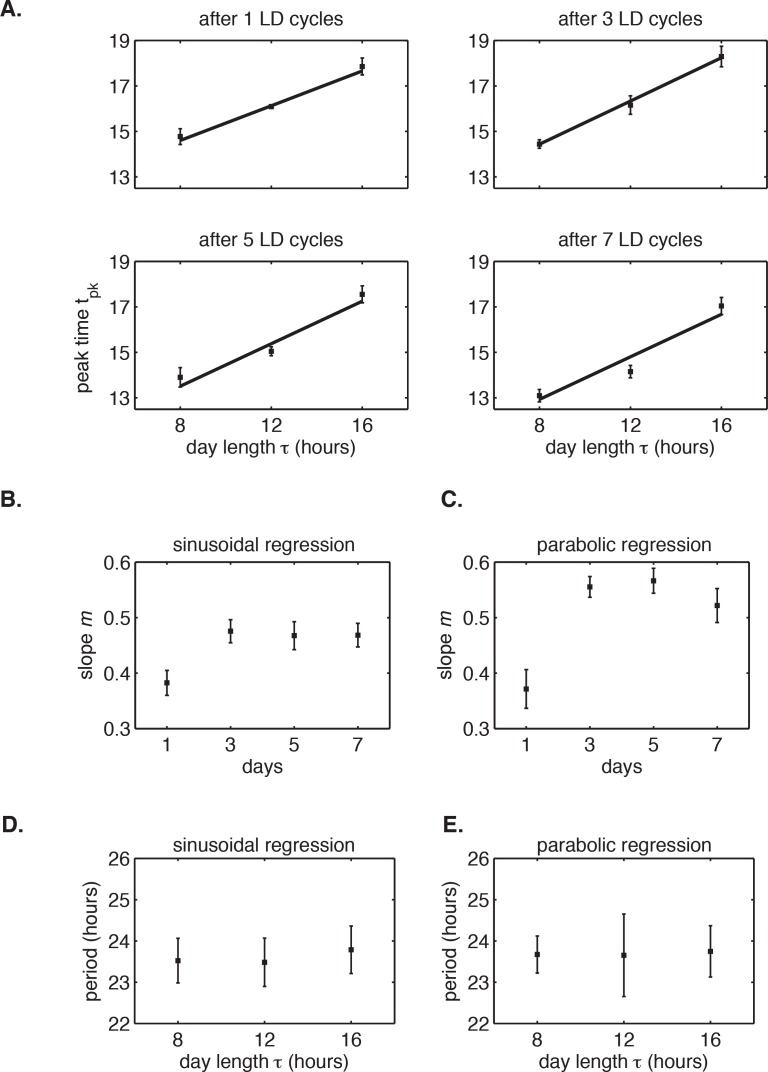
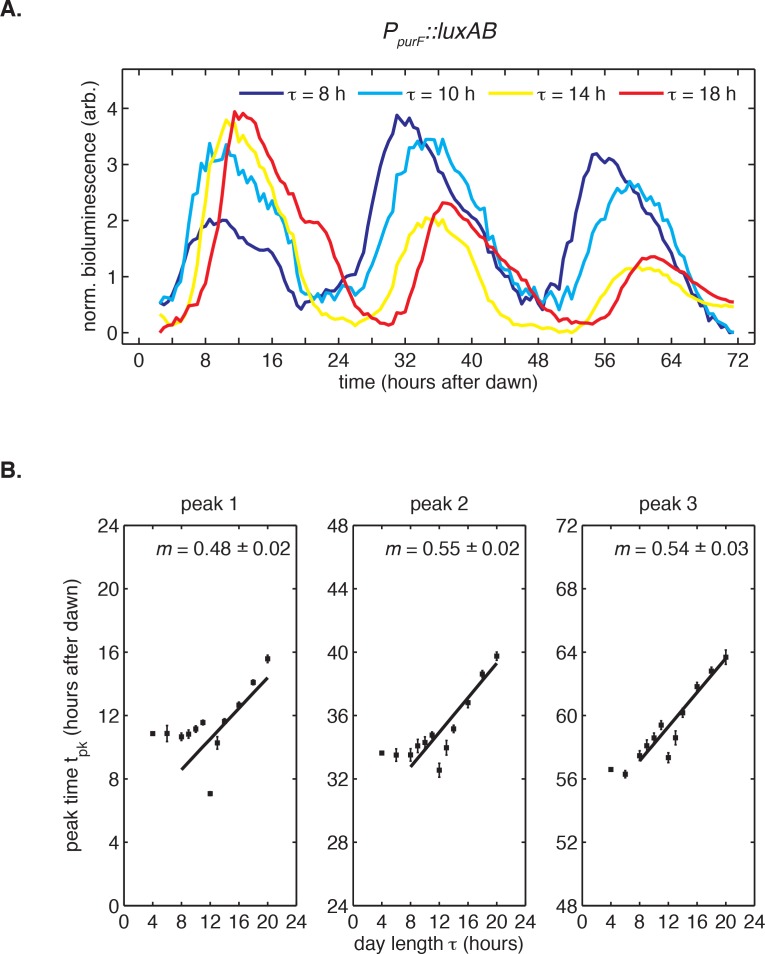
Figure 1. Phase of the cyanobacterial circadian rhythm scales linearly with day length.
(A) LED array device used to grow S. elongatus in programmable light-dark cycles. Cells grown in a 96-well plate on solid media (lower plate, green circles) are illuminated from above by LEDs (red circles). An Arduino microcontroller is used to dynamically change LED intensity in different columns of the plate (inset). Luminescence from the bottom plate is read out every 30 min on a plate reader. Drawing not to scale. (B) Drive-and-release strategy to measure phase of the circadian clock under light-dark (LD) cycling. Cells were exposed to five entraining LD cycles and then released into constant light. Bioluminescence signals (PkaiBC::luxAB) from each well were separated into individual ‘day’ and ‘night’ windows. Data from night portions of the experiment were omitted from analysis (gray bars), and data from the day portions of the experiment were aligned to zero baseline and normalized to unit variance. Dashed lines indicate time of peak reporter signal calculated by parabolic fitting. See Computational methods for details. (C) Peak time of bioluminescence (PkaiBC::luxAB) in light-dark cycles of different day length (red squares) was quantified by local parabolic fitting around the first maximum of the oscillation after release into constant light. Error bars represent standard deviations of peak time estimates from technical replicates (n = 4–8). Slope of the linear fit (red line, m = 0.53 ± 0.01) was determined by linear regression. Dashed and dotted black lines indicate scaling of phase with day length for dawn- and dusk-tracking oscillators; green line indicates midday-tracking behavior.
DOI: http://dx.doi.org/10.7554/eLife.23539.003