Abstract
Mismatch negativity (MMN) is a robustly abnormal brainwave in chronically ill schizophrenia that has generated interest as a disease presence biomarker. Reports of MMN reduction in first-episode schizophrenia have been equivocal, raising uncertainty about its reduction at first psychotic break. Here we tested 29 schizophrenia-spectrum participants under 1 year from their first hospitalization for psychosis and 40 age-, gender-, parental socioeconomic status-, and Wechsler Adult Intelligence Scales III Information-matched healthy controls on both pitch and duration MMN. Participants performed a visual checkerboard tracking task while standard (1kHz, 50ms, 80%), pitch-deviant (1.2kHz, 50ms, 10%) and duration-deviant (1kHz, 100ms, 10%) tones were presented over headphones (75 dB) and EEG was recorded. Independent component analysis was used to remove eye movements and visual stimulus processing activity. Groups did not differ in pitch MMN or duration MMN amplitudes. Smaller pitch and duration MMN amplitudes were associated with lower estimates of premorbid intellect in all participants and independently with greater positive symptoms in first hospitalized schizophrenia. Overall MMN reduction was not present in these relatively high functioning individuals at the first episode of schizophrenia, and therefore is not a good disease presence biomarker for this sample. Future research is warranted to determine the degree of MMN reduction at the first episode of psychosis across a greater range of cognitive impairment, the utility of MMN as an indicator of risk or diagnosis, and its role for understanding pathophysiological mechanisms in emerging psychosis.
Keywords: biomarker, psychosis, first episode, sensory memory, IQ matching
Introduction
Mismatch negativity (MMN) is a small negative EEG deflection elicited outside the focus of attention for changes in stimuli from past patterns. Typically it is elicited by rare sounds differing physically (pitch, duration, loudness, location, etc.) from repeated standard tones. Whether MMN reflects an active comparison of current events with past events,1 a prediction of what should occur,2 or increased sensory responses to rare deviants than to repeated sensory adapted stimuli3 is hotly debated, but not germane to its clinical utility as a possible schizophrenia biomarker. MMN has attracted interest as a biomarker because it is highly abnormal in chronically ill schizophrenia, with effect sizes (Cohen’s d) near 1,4 is relatively easy and inexpensive to measure, and passively acquired.
A biomarker can be used for risk prediction and for screening, diagnosis, or tracking disease progression.5 When used for diagnosis, the putative biomarker should be present in those afflicted, and thus if MMN is a disease presence biomarker, it must obligatorily be reduced at first hospitalization for schizophrenia. The findings in the first episode of schizophrenia literature, however, are equivocal. It is important to note that we are solely dealing with individuals who have experienced psychosis. We make no claim about the MMN prior to psychosis onset and its putative role as a potential screening biomarker. Our question is whether MMN is reduced at the known first episode of psychosis and to what characteristics it might be related.
Here we review pitch and duration MMN findings in the entire first-episode schizophrenia-spectrum and first-episode psychosis (mixed schizophrenia-spectrum and affective-spectrum) literature. In studies that examined only schizophrenia-spectrum psychosis participants within 1 year of their first episode or first hospitalization (table 1), no study reported reduced pitch MMN, with the largest effect a trend-level finding of a small effect size (d = 0.34).17 In studies of mixed first-episode psychosis under 1 year from hospitalization (table 2), there was no evidence of pitch MMN reduction. Based on all extant studies, pitch MMN reduction does not appear suitable as a diagnostic biomarker at first episode.
Table 1.
Summary of First-Episode Schizophrenia-Spectrum MMN Studies
| Study | Time of Testing | N (SzS/HC) | Pitch MMN Reduction | Duration MMN Reduction | IQ or Education Controlled | Effect Size at Fz/Fcz (Cohen’s d) | |
|---|---|---|---|---|---|---|---|
| Tested < 1-y FH | Pitch | Dur. | |||||
| Salisbury et al6,a | <1-y FH | 21/27 | N | Y | 0.09 | ||
| Oknina et al7 | At FH | 25/14 | N | Y | |||
| Oades et al8,b | At FH | 28/22 | Y | N | 1.0 | ||
| Umbricht et al9,c | <6-mo FH | 26/39 | N | N | N | 0.33 | 0.25 |
| (College subsample)c | <6-mo FH | 12/39 | N | N | Y | −0.10 | −0.42 |
| (No college subsample)c | <6-mo FH | 14/39 | Y | Y | N | 0.67 | 0.96 |
| Salisbury et al10,a | <6-mo FH | 20/32 | N | Y | −0.008 | ||
| Devrim-Üçok et al11 | At FH | 30/34 | N | Y | 0.0 | ||
| Magno et al12 | <3-mo FH | 12/27 | N | N | U | −0.36 | −0.24 |
| Kaur et al13,d | <1-y FE | 18/18 | Y | N | 1.47 | ||
| Bodatsch et al14 | At FE | 33/67 | N | Y | U | 0.11 | 0.45 |
| Hsieh et al15 | <1-y FE | 32/56 | Y | N | 0.50 | ||
| Mondragón-Maya et al16,f | At FH | 20/23 | N | Y | 0.17 | ||
| Nagai et al17 | <1-y FE | 20/22 | T | Y | Y | 0.34 | 0.80 |
| Tested < 2-y FH | |||||||
| Kaur et al18,d | First or second episode | 20/20 | Y | N | 1.40 | ||
| Higuchi et al19,e | <2-y FH | 20/20 | Y | U | 1.60 | ||
| Higuchi et al20,e | <2-y FH | 19/19 | Y | U | 1.20 | ||
| Solís-Vivanco et al21,f | <2-y FH | 20/23 | Y | Y | 1.14 | ||
Note: FE, first episode; FH, first hospitalization; HC, healthy controls; IQ, intelligence quotient; MMN, mismatch negativity; SzS, schizophrenia spectrum; Y, yes; N, no; T, trend level; U, unreported. Effect sizes: pitch, pitch MMN (left side); dur., duration MMN (right side). Negative values indicate patient samples had larger responses than healthy subjects.
a–eStudies with matching superscripts present data from overlapping samples. Caution should be used to avoid “double weighting” effect sizes.
cUmbricht et al performed secondary analyses based on whether FHSz had or had not attended college. Note the strong effect on group differences.
fSubject overlap between studies is unclear.
Table 2.
Summary of First-Episode Mixed Psychosis Spectrum MMN Studies
| Study | Time of Testing | N (SzS/APS/HC) | Pitch MMN Reduction | Duration MMN Reduction | IQ or Education Controlled | Effect Size at Fz/Fcz (Cohen’s d) | |
|---|---|---|---|---|---|---|---|
| Tested < 1-y FH | Pitch | Dur. | |||||
| Valkonen-Korhonen et al22 | <1-y FH | 21/4/19 | N | Y | 0.05 | ||
| Hermens et al23,a | <1-y FE | 9/8/17 | Y | N | 0.86 | ||
| Atkinson et al24,b | At FE | U 10/20 | Y | N | Short | 1.00 | |
| Long | 1.52 | ||||||
| Kaur et al25,a | U | 27/33/30 | N | Y | 0.39 | ||
Note: Abbreviations are explained in the first footnote to table 1. APS, affective psychosis spectrum.
aSubject overlap between matching superscripts.
bDiagnostic breakdown unknown, all psychotic subjects included. Both short- and long-duration deviants were tested.
The results for duration MMN reduction are variable. Among studies that examined only schizophrenia-spectrum psychosis participants within 1 year of their first episode or first hospitalization (table 1), most showed reduction. However, effect sizes ranged from negative (d = −0.42, patients showing a larger MMN than controls) to large (d = 1.47, a deficit larger than in long-term schizophrenia). An important clue to what might be at least partially driving the variability in duration MMN findings was provided by a follow-up analysis by Umbricht et al.9 Although no duration MMN reduction was observed in the entire group of first-episode schizophrenia (FESz) subjects, patients who had not attended college had smaller MMNs than controls and first break patients that did. This suggests that academic achievement and, by inference, premorbid intelligence quotient (IQ) might be important variables associated with the equivocal duration MMN findings. In studies of the early course of the schizophrenia spectrum (including subjects >1 y postbreak), large effects were reported (table 1). In studies of mixed first-episode psychosis under 1 year from hospitalization (table 2), 2 reported duration MMN reduction, but did not control for IQ or education, while the one that did25 reported no significant duration MMN reduction at Fz. In summary, there is some evidence for duration MMN reductions at the first break of schizophrenia, but results are equivocal, and may be related to intellectual deficits.
It is unclear whether MMN reflects disease presence (risk, screening, and diagnosis) or progression (pathology subsequent to psychosis onset). The 3 studies with a longitudinal component show no significant reduction at first episode, with MMN deficits developing shortly thereafter. Salisbury et al10 replicated no reduction of pitch-deviant MMN at first hospitalization in 20 first hospitalized schizophrenia participants partially overlapping with Salisbury et al6; but patients with the most impaired MMN had the greatest gray matter loss in left auditory cortex (Heschl’s gyrus). Pitch MMN showed significant reductions at follow-up ~1.5 years later, associated with progressive left auditory cortex gray matter volume loss. All comparisons were made between groups matched for estimated premorbid IQ. Devrim-Üçok et al11 reported no significant reductions in pitch MMN in 30 FESz participants (d = 0.0), although they saw MMN reductions in the postacute phase 1–9 months later (d = 0.43). Premorbid IQ was not reported, but groups were matched for years of education. Kaur et al25 studied 15 affective-spectrum and 12 schizophrenia-spectrum subjects. When this mixed first-episode psychosis group and healthy controls were matched for cognitive functioning, no significant MMN reductions at first episode were found at Fz, and progressive MMN reductions with course were detected. Consistent with possible progressive MMN decline in the immediate postbreak period, largest effect sizes for MMN reduction were observed in samples including patients over 1 year from first psychosis (table 1).
MMN reduction at first episode is associated with poor educational achievement9 and loss of cortical gray matter in auditory cortex.10 These data suggest that, although the group mean of MMN may be normal, there are likely certain FESz subjects with reduced MMNs, auditory cortex gray matter loss, and poor functioning and achievement. (Parenthetically, chronic schizophrenia patients that did not finish high school show the greatest MMN impairments, particularly for duration MMN26; see their figure 4.) It is imperative to determine whether MMN is sensitive to psychosis presence or rather tracks progressive perionset brain gray matter loss. The purpose of this study was to examine pitch and duration MMN in a new sample of first-episode schizophrenia-spectrum patients and healthy controls to assess whether MMN was reduced in first-episode patients and showed associations with specific symptoms and estimates of premorbid intellect.
Methods
Subjects
Subjects had no history of a learning disability, including dyslexia, special education, childhood treatment for attention deficit disorder/attention deficit hyperactivity disorder, any infectious or neurological disease affecting the central nervous system, any loss of consciousness >20 minutes and/or traumatic brain injury with sequelae, electroconvulsive therapy, drug or alcohol detox or dependence within the last 5 years, intravenous drug abuse ever, or seizure disorder. Subjects had a minimum 9th grade education and an estimated IQ >85 as ascertained from chart notes. All subjects had normal hearing as assessed with audiometry, defined as within 30 dB nHL, no more than 15 dB difference between ears at 500, 1000, and 1500 Hz.
Patients were recruited from consecutive inpatient admissions at McLean Hospital less than 1 year from their first inpatient admission for psychosis (the mean since first hospitalization was 9.6 [14.5] wk, the median was 2.9wk). All patients received a research diagnosis based on the Structured Clinical Interview for the Diagnostic and Statistical Manual (SCID)—Patient edition and/or chart review. The first hospitalized schizophrenia-spectrum group (FHSz, n = 29) included schizophrenia (17 paranoid, 1 disorganized, 2 undifferentiated), schizophreniform disorder (2), delusional disorder (1), and schizoaffective disorder (depressed subtype 1, bipolar subtype 5). Fourteen FHSz received >6 months follow-up diagnoses. Two FHSz were unmedicated. The remaining FHSz were on antipsychotic medications (the mean lifetime exposure to antipsychotic medications was 9.5 [14.5] wk, the median was 2.7wk). Psychiatrically healthy control subjects (HC, n = 40) were recruited from newspaper advertisements, and screened using the SCID Non-Patient Edition P and SCID II. No HC reported an Axis I psychiatric disorder in a first-degree relative. The McLean Hospital IRB approved this study. After complete description of the study, written informed consent was obtained. Subjects were paid $15/h for their participation. All participants performed the Information, Vocabulary, Digit Span, and Digit Symbol Subtests of the Weschsler Adult Intelligence Scales—3rd Edition (WAIS III), and the Mini-Mental State Examination (MMSE). Patient symptoms were rated on the Positive and Negative Syndrome Scale (PANSS), Scale for the Assessment of Positive Symptoms (SAPS), and Scale for the Assessment of Negative Symptoms (SANS).
Procedure
Subjects were presented binaural tone pips. Standard tones (1kHz, 75 dB, 50ms duration, 10ms rise/fall) were presented on 80% of trials. Pitch-deviant tones (1.2kHz, 75 dB, 50ms duration, 10ms rise/fall) and duration deviants (1kHz, 75 dB, 100ms duration, 10ms rise/fall) were presented on 10% of trials each. Tones were presented with a 300ms stimulus onset asynchrony while subjects performed a visual task. Subjects sat 1 m from a monitor on which was displayed a checkerboard with green and red squares, and instructed to ignore the tones, pay attention to the checkerboard, and make a right index finger keypad response to checkerboard color reversals (asynchronous, range: 430–1500ms). Tracking performance was monitored, and subjects were encouraged to maintain the task.
EEG Recording
EEG activity was recorded from 60 scalp sites and the nose tip using a 64-channel cap (custom designed Electro-Cap International sintered Ag–AgCl caps using the 10–10 system). Activity was recorded continuously using SynAmps and Scan Acquire (Neuroscan/Compumedics USA). The right mastoid served as the recording reference, except for 2 bipolar electro-oculogram channels. Two electrodes medial to the right eye, one above and one below, monitored vertical eye movements and blinks. Electrodes at the outer canthi of the eyes monitored horizontal eye movements. The forehead served as ground. Electrode impedances were below 5 kOhms. The EEG bandpass was 0.10 (6 dB/octave roll-off) to 100 Hz (24 dB/octave roll-off). EEG was digitized at 500 Hz.
EEG Preprocessing
EEG data were preprocessed using custom MATLAB scripts utilizing the extended infomax independent component analysis (ICA) algorithm27 in EEGLAB.28 EEG data were bandpassed (0.2–100 Hz), notch filtered (58–62 Hz), and segmented into 800ms epochs centered on auditory stimulus onset. Bad channels and epochs containing unique nonstereotyped artifacts were removed. The ICA components capturing stereotyped artifacts were excluded.29,30 ICA was also employed to remove residual checkerboard visual activity. Segments containing artifacts were eliminated (±50 μV).
MMN Processing
Data was re-referenced to the nose tip. Standard, pitch-deviant, and duration-deviant waveforms were constructed by averaging reprojected EEG epochs (−50 to 350ms relative to tone onset). MMN was visualized by subtraction of the standard waveform from the deviant waveform. Pitch MMN was measured over 120–220ms. Duration-deviant MMN was measured over 170–270. These intervals were based on the grand-averaged morphology, and captured the entire MMN ERP and the mastoid and frontal peaks well.6,31 MMNs were subjected to current source density (CSD) analysis to show source–sink topography. Interpolation was done using spherical splines, with the order of splines set to 4, the maximum degree of Legendre polynomials set to 10, and a default lambda of 1e-5.
Statistical Analyses
Demographics and basic cognitive measures were compared between groups with t tests and chi-squared analyses where appropriate. Pitch MMN and duration MMN were analyzed in 2 ways. First, to maximize the probability of finding a significant difference, a t test between groups at Fz was conducted. Second, a regional analysis of left and right frontocentral sites was performed (F1/2, FC1/2, and C1/2). Correlations between MMNs at Fz and basic cognitive measures and symptoms were conducted using Pearson’s r. Results were considered significant at P ≤ .05.
Results
Groups were matched for age, gender, handedness, parental socioeconomic status (SES), and WAIS III Information scaled scores. (Five patients refused to complete neuropsychological testing.) Consistent with their illness, patients has significantly lower SES and scored significantly lower on the MMSE and WAIS III Digit Span and Digit Symbol, with trend-level impairment on Vocabulary (table 3). Note that WAIS III Information and Vocabulary were above average in FHSz, whereas working memory subtests of Digit Span and Symbol-Digit were below average (mean WAIS scaled scores are 10, SD is 3). Clinical measures are presented in table 4. Amplitudes at each site tested and mastoids are presented in table 5.
Table 3.
Demographics and Cognitive Measures
| FHSz | HC | Statistics | |
|---|---|---|---|
| Group size | 29 | 40 | |
| Age | 25.0 (8.6) | 24.9 (7.4) | t 67 = 0.06, P = .95 |
| Gender | 20M/9F | 29M/11F | χ2 = 0.17, P = .68 |
| Handedness | 0.60 (0.51) | 0.76 (0.30) | t 62 = 1.58, P = .16 |
| SES | 3.3 (1.4) | 1.9 (0.6) | t 65 = 5.31, P < .001 |
| PSES | 1.5 (0.8) | 1.7 (0.9) | t 62 = 0.75, P = .46 |
| Education (y) | 13.1 (2.0) | 15.0 (1.4) | t 65 = 4.50, P < .001 |
| MMSE | 28.5 (1.4) | 29.4 (0.7) | t 62 = 3.21, P = .002 |
| WAIS Information | 12.7 (2.5) | 13.1 (2.2) | t 62 = 0.77, P = .44 |
| WAIS Vocabulary | 13.0 (3.0) | 14.3 (2.8) | t 62 = 1.77, P = .08 |
| WAIS Digit Span | 9.6 (2.2) | 12.4 (2.3) | t 62 = 4.81, P < .001 |
| WAIS Digit Symbola | 7.5 (2.3) | 11.5 (2.7) | t 61 = 6.09, P < .001 |
Note: Values are mean (SD) unless otherwise indicated. WAIS scaled scores. Significant differences between groups are highlighted in bold. PSES, parental socioeconomic status; FHSz, first hospitalized schizophrenia; HC, healthy controls; MMSE, Mini-Mental State Examination; SES, socioeconomic status; WAIS, Weschsler Adult Intelligence Scales.
aWAIS Digit Symbol data was lost for 1 HC. Variations in df reflect missing values.
Table 4.
Clinical Measures
| PANSS Total | 78.3 (16.3) |
| PANSS Positive | 19.7 (4.4) |
| PANSS Negative | 17.9 (7.6) |
| PANSS Composite | 1.8 (9.1) |
| PANSS Thought Disturbance | 10.7 (3.7) |
| PANSS Paranoia/Belligerence | 8.1 (3.0) |
| PANSS General | 36.5 (8.4) |
| PANSS Anergia | 10.6 (4.0) |
| PANSS Activation | 4.8 (1.3) |
| PANSS Depression | 10.3 (4.0) |
| SAPS | 8.7 (4.2) |
| SANS | 9.8 (4.2) |
| Medication (CPZ equivalents) | 267.1 (170.3) |
Note: Values are mean (SD). CPZ, chlorpromazine; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Table 5.
MMN Amplitudes
| Site | FHS | HC | t Test | Effect Size (d) |
|---|---|---|---|---|
| Pitch | ||||
| F1 | −1.8 (2.2) | −1.6 (1.7) | t 67 = 0.53, P = .60 | −0.10 |
| Fz | −1.9 (2.2) | −1.7 (1.7) | t 67 = 0.42, P = .68 | −0.10 |
| F2 | −1.8 (2.2) | −1.7 (1.7) | t 67 = 0.37, P = .71 | −0.05 |
| FC1 | −2.0 (2.4) | −1.7 (1.8) | t 67 = 0.57, P = .57 | −0.14 |
| FC2 | −1.9 (2.3) | −1.7 (1.9) | t 67 = 0.38, P = .71 | −0.09 |
| C1 | −1.8 (2.3) | −1.5 (1.7) | t 67 = 0.74, P = .46 | −0.15 |
| Cz | −1.9 (2.3) | −1.7 (1.8) | t 67 = 0.42, P = .67 | −0.10 |
| C2 | −1.9 (2.3) | −1.7 (1.9) | t 67 = 0.40, P = .69 | −0.09 |
| M1 | 0.8 (1.3) | 1.2 (1.2) | t 67 = 1.34, P = .19 | 0.32 |
| M2 | 0.8 (1.6) | 1.2 (1.2) | t 67 = 1.19, P = .24 | 0.28 |
| Duration | ||||
| F1 | −2.1 (1.9) | −2.3 (1.7) | t 67 = 0.54, P = .59 | 0.11 |
| Fz | −2.2 (1.9) | −2.4 (1.7) | t 67 = 0.46, P = .65 | 0.11 |
| F2 | −2.3 (1.9) | −2.4 (1.7) | t 67 = 0.33, P = .75 | 0.06 |
| FC1 | −2.3 (1.9) | −2.6 (1.9) | t 67 = 0.64, P = .52 | 0.16 |
| FC2 | −2.3 (2.0) | −2.6 (1.8) | t 67 = 0.64, P = .53 | 0.16 |
| C1 | −2.0 (2.0) | −2.3 (1.9) | t 67 = 0.62, P = .54 | 0.15 |
| Cz | −2.1 (2.0) | −2.6 (1.9) | t 67 = 0.88, P = .38 | 0.26 |
| C2 | −2.2 (2.0) | −2.6 (1.9) | t 67 = 0.83, P = .41 | 0.21 |
| M1 | 1.2 (1.0) | 1.1 (1.5) | t 67 = 0.32, P = .75 | −0.08 |
| M2 | 1.3 (1.2) | 1.2 (1.8) | t 67 = 0.20, P = .84 | −0.07 |
Note: Values are mean μV (SD). FHS, first hospitalized schizophrenia; HC, healthy control; MMN, mismatch negativity.
Pitch MMN
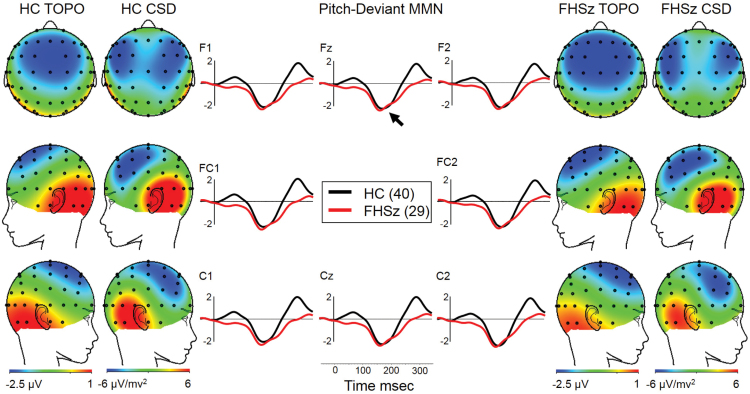
Both groups showed MMN to pitch deviants with frontocentral voltage topography and CSD topography consistent with bilateral temporal generators (figure 1). Groups did not differ in pitch MMN at Fz (table 5). FHSz were slightly larger than HC (small effect size, d = −0.10). Frontocentral analysis likewise revealed no significant group differences (F1,67 = 0.25, P = .62, partial η2 = 0.004).
Fig. 1.
Pitch-deviant MMN (arrow at Fz in middle panel). Voltage topography is highly similar in both groups (HC TOPO and FHSz TOPO) and CSD maps are consistent in both groups with bilateral temporal lobe generators. CSD, current source density; FHSz, first hospitalized schizophrenia; HC, healthy controls; MMN, mismatch negativity; TOPO, topography.
Duration MMN
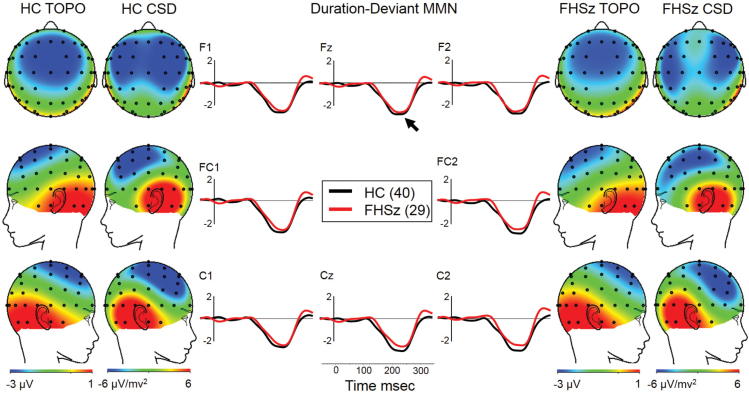
Both groups showed MMN to duration deviants with frontocentral voltage topography and CSD topography consistent with bilateral temporal generators (figure 2), albeit of a slightly different orientation that pitch MMN (figure 1). Groups did not differ in duration MMN at Fz (table 5). FHSz were slightly smaller than HC (small effect size, d = 0.11). Frontocentral analysis likewise revealed no significant group differences (F1,67 = 0.38, P = .54, partial η2 = 0.006).
Fig. 2.
Duration-deviant MMN (arrow at Fz in middle panel). Voltage topography is highly similar in both groups (HC TOPO and FHSz TOPO) and CSD maps are consistent in both groups with bilateral temporal lobe generators. CSD, current source density; FHSz, first hospitalized schizophrenia; HC, healthy controls; MMN, mismatch negativity; TOPO, topography.
Cognitive and Clinical Correlations
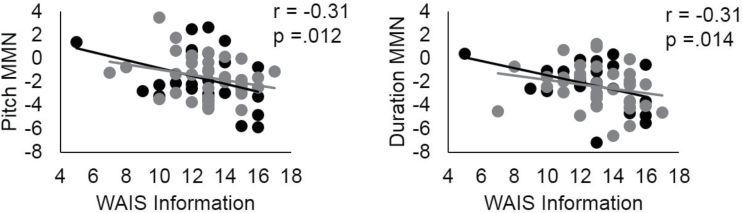
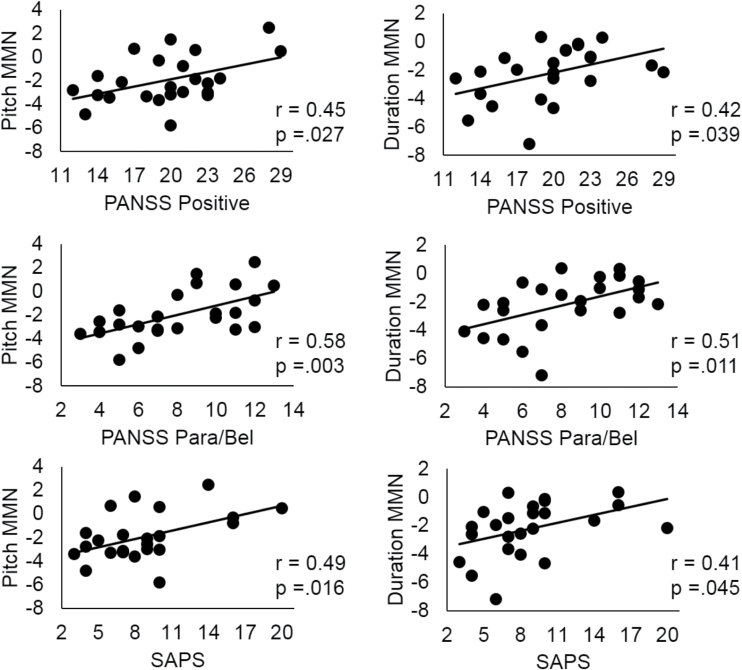
Among all subjects, there was a significant association between WAIS Information scaled score and both pitch (r = −.31, P = .012) and duration MMN at Fz (r = −.31, P = .014, figure 3). The more negative (larger) the MMN, the more positive (greater) the Information scaled score. In patients, significant associations were observed between pitch and duration MMN at Fz and PANSS and SAPS positive symptom factors, and the PANSS Paranoia/Belligerence factor (see r values in figure 4), where more severe symptoms were associated with smaller MMNs. These associations remained after controlling for premorbid intellect.
Fig. 3.
Correlations between pitch-deviant and duration-deviant MMN at Fz and WAIS III Information scaled score in all participants. Black: first hospitalized schizophrenia; gray: healthy controls. MMN, mismatch negativity; WAIS III, Weschsler Adult Intelligence Scales—3rd Edition.
Fig. 4.
Correlations between reduced pitch-deviant MMN and duration-deviant MMN at Fz and positive symptoms at first hospitalization, particularly for paranoia. MMN, mismatch negativity; PANSS Para/Bel, Positive and Negative Syndrome Scale Paranoia/Belligerence Factor; SAPS, Scale for the Assessment of Positive Symptoms.
Sampling Simulation and Premorbid IQ Effects
To determine whether unmatched premorbid IQ could affect the findings, we performed a median split for WAIS III Information scaled scores in each group (median = 13 for both groups) and compared the lower FESz to the higher HC. WAIS Info was significantly reduced (FHSz: 10.5 [2.2], HC: 15.1 [0.7], t25 = 7.8, P < .001). Pitch MMN was not significantly reduced at Fz (FHSz: −1.0 [2.1], HC: −2.0 [1.0], t25 = 1.6, P = .118), but duration MMN was (FHSz: −1.6 [1.1], HC: −3.1 [1.7], t25 = 2.6, P < .016).
Discussion
Neither pitch MMN nor duration MMN were reduced in FHSz within 1 year of first hospitalization (most within 3wk), when matched for estimated premorbid IQ with HC. Despite significant symptoms and cognitive impairment in Digit Span and Digit Symbol performance, MMN remained within normal limits in this above-average sample. Pitch and duration MMN were smallest in FHSz showing the greatest positive symptoms and paranoia. Hence, the underlying pathology related to symptom severity is reflected in pathophysiology of MMN generators. However, in this group overall, FHSz MMN distributions overlapped HC; no patient was smaller (more positive) than the worst HC (figure 3).
The association between premorbid intellect and MMN amplitude may provide an explanation for inconsistent findings of reduced duration MMN in FESz; our median split simulation, where FHSz had a 4.4 point reduction in WAIS Information scaled scores, showed reduced duration but not pitch MMN, similar to the existing aggregate MMN findings (table 1). This begs the question of upon what measure(s) to match groups. Educational achievement, social and occupational functioning, and cognitive functioning are all affected by the disease, leading to the so-called “matching fallacy.”32 Cognitive deficit remains a strong and perhaps the best predictor of functional outcome,33–36 even at first hospitalization.37 If the disease affects cognition, but cognition is associated independently with the measure of interest, it is difficult to “un-confound” cognition and disease effects.
Not to match subjects groups on some estimate of premorbid IQ risks ascribing differences to the disease process better accounted for by a priori intellectual differences. Showing that intellectually impaired FESz show smaller MMN does not necessarily imply that MMN is specifically reduced in FESz (see study by Chapman and Chapman38). Research has identified so-called “hold” variables that are more resistant to schizophrenia,39–42 typically including WAIS Information and Vocabulary subtests and the Wide Range Achievement Test (WRAT) reading and spelling subtests. Typically, tests of semantic or “crystallized” knowledge serve as “hold” variables, while tests of executive function, working memory, or “fluid” intelligence are highly impaired. For example, in our sample where WAIS Information was matched, robust deficits were observed in WAIS Digit Span and Digit Symbol measures (table 3). To the extent that WAIS Information scaled scores are relatively resistant to schizophrenia, they are useful for avoiding ascribing deficits specifically to the disease better accounted for by premorbid intellectual differences.
On the other hand, it is not clear that “hold” tests are impervious to psychosis. Deficits in WAIS Information and Vocabulary (~2 scaled points) have been reported in first-episode samples.43,44 Matching for WAIS Information may underestimate premorbid intellectual function in FHSz. To closely approximate the smaller decline in WAIS Information observed in epidemiologic studies, we compared 15 FHSz (WAIS Info: 11.3 [2.2]) to 15 HC (WAIS Info: 14.5 [1.3], t28 = 4.8, P < .001). Neither pitch (t28 = 0.18, P = .53) nor duration MMN (t28 = 1.2, P = .26) were reduced. These simulation data suggest that even in these high normal FHSz, extreme differences in premorbid IQ may influence finding reduced MMN, while differences closer to the population norm do not reveal MMN reductions.
If WAIS Information was independently affected by the disease, then the correlation with MMN in FHSz should be greater than in HC (due to additive or multiplicative effects of disease on the IQ-MMN association). There was no significant difference between the correlation in FHSz and HC for pitch (Fisher’s z = 0.242, P ≈ .80) or duration MMN (Fisher’s z = 0.696, P ≈ .48). Still it is likely that some effects of disease are removed when matching for estimates of premorbid IQ, and the field needs to develop procedures to disentangle the confound.
Researchers are faced with a difficult choice. While matching for full-scale IQ or for years of education may be erroneously selecting for higher premorbid IQ in patients (perpetuating a “matching fallacy”),45 group differences in overall premorbid IQ independent from disease may spuriously select for group differences in the dependent variable. Here IQ accounted for approximately 10–16% of the MMN variance (r2; pitch, duration, respectively) with positive symptoms accounting for an additional 13–46% of the variance. While MMN pathophysiology should provide clues about underlying pathology reflected in positive symptoms at first break, in this relatively high functioning sample of FHSZ it was not useful for diagnosis. Association with premorbid intellect cannot account for all reported differences, yet may be a major cause of equivocal results. Future research is warranted to determine the degree of MMN reduction at the first episode of psychosis across a greater range of cognitive impairment, the utility of MMN as an indicator of risk or diagnosis, and its role for understanding pathophysiological mechanisms in emerging psychosis.
Inspection of table 1 of every MMN study within a year of first psychosis shows little effect for pitch MMN, and a small to medium effect for duration MMN. We hypothesize that in FHSz without marked intellectual decline MMN deficits with a large effect size emerge very shortly after the onset of psychosis.10,11,25 The first-episode subgroup that showed MMN reductions in Umbricht et al9 may have been suffering from longer untreated psychosis and more progressive cortical gray matter loss in auditory cortex.10,42,46 Belger and colleagues47,48 have presented evidence suggesting MMN tracks progressive deficits early in disease course. While these data suggest that MMN is a biomarker well suited to track disease progression, further longitudinal studies must be conducted to replicate and expand the extant data.
In particular, research must be undertaken to assess the relationship between accurate measure of psychosis onset and MMN reductions; the lack is a weakness of the current study. It is also a limitation that half of the subjects did not return for diagnostic follow-up, an issue which plagues most first-episode studies. It is possible that the patients studied here express greater intelligence or neurocognitive robustness than the norm, representing only a subgroup of the larger FHSz population. Larger samples with a wider range of premorbid intellect are needed. We make no claim regarding the use of MMN as a screening tool for incipient psychosis. We have no data on such cases, and will not argue beyond our data. It is entirely possible that intensive treatment at first inpatient hospitalization may improve the MMN for the short term, or that compensatory cortical gain mechanisms at first psychosis may increase MMN within a prodromal context of reduction. Only long-term longitudinal studies of at-risk individuals comprising converters and nonconverters can answer such questions.
Consistent with prior studies, reduced MMN correlated with increased positive symptoms. Fisher and colleagues49,50 showed correlations between MMN and the severity of the specific positive symptom of auditory verbal hallucinations (AVH). Our data pointed to a more general relationship of MMN and positive symptoms, as well as with paranoia. These data suggest that the symptom profile at first hospitalization for schizophrenia may be weighted toward paranoia and delusions, and more stable associations between MMN and AVH may develop with disease course. Here the associations between MMN and positive symptoms remained when partialing out the contribution of premorbid intellect. Thus MMN is likely to provide valuable information regarding cortical mechanisms underlying positive symptoms.
In summary, we saw no pitch or duration MMN reductions in FHSz that were matched with HC on WAIS Information, used as a proxy estimate of premorbid IQ. However, for both groups, reduced MMN correlated with lower WAIS information subtest scores. In addition, for patients, smaller MMNs correlated with positive symptom severity, independent of premorbid IQ estimate. Premorbid intellectual functioning may be a confounding variable at least partially underlying the equivocal reports of duration MMN reduction at first schizophrenic break, as some studies accounted for it but others did not. Carefully controlled longitudinal studies are warranted to determine precisely what contribution MMN can make to risk assessment, screening, and diagnosis.
Funding
National Institutes of Health (R01 MH40799 to R.W.M. and R01 MH094328 to D.F.S.).
Acknowledgments
We thank W. Iacono and G. Miller for helpful editorial comments. Data were collected by D.F.S. while faculty at Harvard Medical School and analyzed at the University of Pittsburgh. The authors have no conflicts of interest and take responsibility for the integrity and accuracy of the report.
References
- 1. Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other measures of cognitive function. Behav Brain Sci. 1990;13:201–288. [Google Scholar]
- 2. Winkler I. Interpreting the mismatch negativity. J Psychophysiol. 2007;21:147–163. [Google Scholar]
- 3. May PJ, Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47:66–122. [DOI] [PubMed] [Google Scholar]
- 4. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 5. Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. [DOI] [PubMed] [Google Scholar]
- 7. Oknina LB, Wild-Wall N, Oades RD, et al. Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophr Res. 2005;76:25–41. [DOI] [PubMed] [Google Scholar]
- 8. Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Röpcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. [DOI] [PubMed] [Google Scholar]
- 10. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devrim-Uçok M, Keskin-Ergen HY, Uçok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:179–185. [DOI] [PubMed] [Google Scholar]
- 12. Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008;64:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF. MMN/P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res. 2011;130:203–209. [DOI] [PubMed] [Google Scholar]
- 14. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh MH, Shan JC, Huang WL, et al. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr Res. 2012;140:243–249. [DOI] [PubMed] [Google Scholar]
- 16. Mondragón-Maya A, Solís-Vivanco R, León-Ortiz P, et al. Reduced P3a amplitudes in antipsychotic naïve first-episode psychosis patients and individuals at clinical high-risk for psychosis. J Psychiatr Res. 2013;47:755–761. [DOI] [PubMed] [Google Scholar]
- 17. Nagai T, Tada M, Kirihara K, et al. Auditory mismatch negativity and P3a in response to duration and frequency changes in the early stages of psychosis. Schizophr Res. 2013;150:547–554. [DOI] [PubMed] [Google Scholar]
- 18. Kaur M, Battisti RA, Lagopoulos J, Ward PB, Hickie IB, Hermens DF. Neurophysiological biomarkers support bipolar-spectrum disorders within psychosis cluster. J Psychiatry Neurosci. 2012;37:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8:e54080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T. Mismatch negativity and p3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Front Behav Neurosci. 2014;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solís-Vivanco R, Mondragón-Maya A, León-Ortiz P, Rodríguez-Agudelo Y, Cadenhead KS, de la Fuente-Sandoval C. Mismatch negativity reduction in the left cortical regions in first-episode psychosis and in individuals at ultra high-risk for psychosis. Schizophr Res. 2014;158:58–63. [DOI] [PubMed] [Google Scholar]
- 22. Valkonen-Korhonen M, Purhonen M, Tarkka IM, et al. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res Cogn Brain Res. 2003;17:747–758. [DOI] [PubMed] [Google Scholar]
- 23. Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. [DOI] [PubMed] [Google Scholar]
- 24. Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. [DOI] [PubMed] [Google Scholar]
- 25. Kaur M, Lagopoulos J, Lee RS, et al. Longitudinal associations between mismatch negativity and disability in early schizophrenia- and affective-spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:161–169. [DOI] [PubMed] [Google Scholar]
- 26. Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. [DOI] [PubMed] [Google Scholar]
- 28. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- 29. Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- 30. Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. Neuroimage. 2010;49:2248–2263. [DOI] [PubMed] [Google Scholar]
- 31. Hirayasu Y, Potts GF, O’Donnell BF, et al. Auditory mismatch negativity in schizophrenia: topographic evaluation with a high-density recording montage. Am J Psychiatry. 1998;155:1281–1284. [DOI] [PubMed] [Google Scholar]
- 32. Meehl PE. High school yearbooks: a reply to Schwarz. J Abnorm Psychol. 1971;77:143–148. [DOI] [PubMed] [Google Scholar]
- 33. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 34. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 35. González-Blanch C, Perez-Iglesias R, Pardo-García G, et al. Prognostic value of cognitive functioning for global functional recovery in first-episode schizophrenia. Psychol Med. 2010;40:935–944. [DOI] [PubMed] [Google Scholar]
- 36. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 37. Vesterager L, Christensen TØ, Olsen BB, et al. Cognitive and clinical predictors of functional capacity in patients with first episode schizophrenia. Schizophr Res. 2012;141:251–256. [DOI] [PubMed] [Google Scholar]
- 38. Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14:303–311. [DOI] [PubMed] [Google Scholar]
- 39. Bilder RM, Degreef G, Pandurangi AK, Rieder RO, Sackeim HA, Mukherjee S. Neuropsychological deterioration and CT scan findings in chronic schizophrenia. Schizophr Res. 1988;1:37–45. [DOI] [PubMed] [Google Scholar]
- 40. Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT. The “3 Rs” and neuropsychological function in schizophrenia: an empirical test of the “matching fallacy”. Neuropsychology. 1996;10:22–31. [DOI] [PubMed] [Google Scholar]
- 41. Morice R, Delahunty A. Frontal/executive impairments in schizophrenia. Schizophr Bull. 1996;22:125–137. [DOI] [PubMed] [Google Scholar]
- 42. Amminger GP, Edwards J, Brewer WJ, Harrigan S, McGorry PD. Duration of untreated psychosis and cognitive deterioration in first-episode schizophrenia. Schizophr Res. 2002;54:223–230. [DOI] [PubMed] [Google Scholar]
- 43. Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull. 1992;18:437–448. [DOI] [PubMed] [Google Scholar]
- 44. González-Blanch C, Crespo-Facorro B, Alvarez-Jiménez M, et al. Cognitive dimensions in first-episode schizophrenia spectrum disorders. J Psychiatr Res. 2007;41:968–977. [DOI] [PubMed] [Google Scholar]
- 45. Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109:743–752. [DOI] [PubMed] [Google Scholar]
- 46. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van der Stelt O, Belger A. Application of electroencephalography to the study of cognitive and brain functions in schizophrenia. Schizophr Bull. 2007;33:955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Belger A, Yucel GH, Donkers FC. In search of psychosis biomarkers in high-risk populations: is the mismatch negativity the one we’ve been waiting for? Biol Psychiatry. 2012;71:94–95. [DOI] [PubMed] [Google Scholar]
- 49. Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119:909–921. [DOI] [PubMed] [Google Scholar]
- 50. Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’ multi-feature paradigm. Int J Psychophysiol. 2011;81:245–251. [DOI] [PubMed] [Google Scholar]