Figure 13.
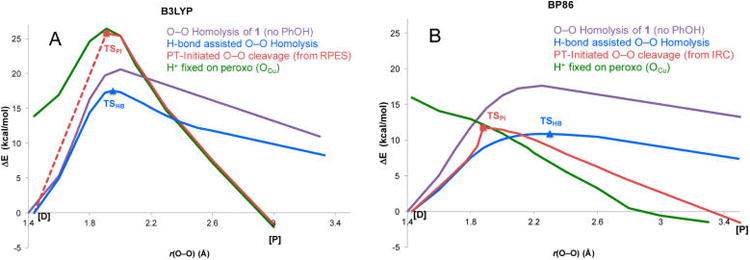
Comparison of B3LYP and BP86 PESs for the O−O homolysis without phenol (purple), PI pathway (red), H-bond assisted O−O homolysis (blue), and the phenolic H+ transferred and optimized on the peroxo (green) vs OFe−OCu separation. Note that the TSPI in B3LYP was obtained by increasing and fixing the OCu−OPh distance to 2.6 Å to prevent the H+ from returning to the phenolate, as an unconstrained transition state search converged to the much lower energy TSHB. Changing the OCu−OPh distance was found to have only a minor effect on the energy when the phenol was deprotonated. The dotted line denotes that the relaxed PES through TSPI requires an additional structural constraint and therefore is not generated from an IRC.
