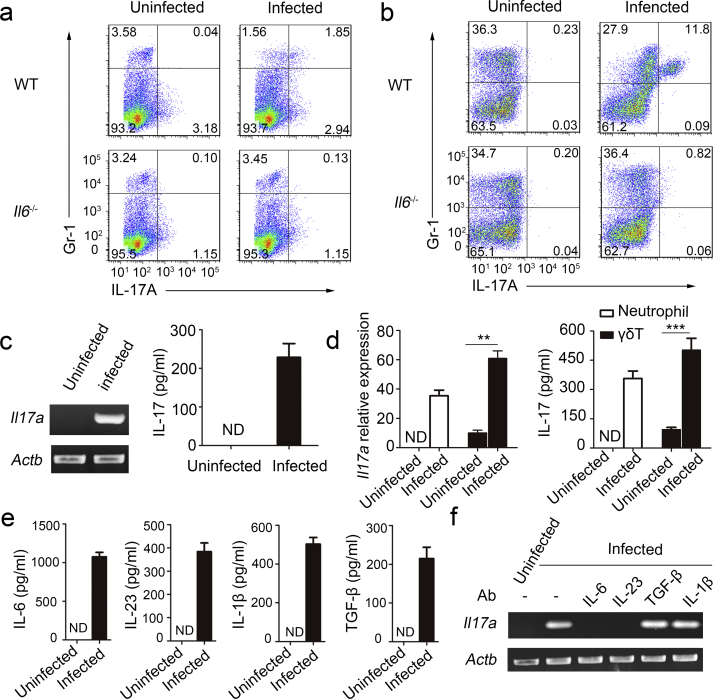
Fig. 1.
Induction of IL-17-producing neutrophils during MTB H37Rv infection. Wild type (WT) and Il6−/− mice were intratracheally (i.t.) infected with MTB H37Rv for three days. (a) Splenocytes were incubated with anti-Gr-1, permeabilized and incubated with anti-IL-17, and examined by flow cytometry. Numbers in quadrants indicate percent cells in each throughout. (b) Bone marrow cells were incubated in RPMI 1640 medium with splenocyte supernatant from the same mouse. And then after 18 h, these cells were incubated with anti-Gr-1, permeabilized and incubated with anti-IL-17, and examined by flow cytometry. (c) Purified neutrophils from total bone marrow cells of infected or uninfected WT mice were incubated in RPMI 1640 medium with splenocyte supernatant from the same mouse. Il17a gene expression in purified neutrophil populations was examined by PCR. PCR products were qualitatively examined on 1% agarose gel. Concentration of IL-17 in supernatant was detected by ELISA. (d) Purified neutrophils and γδT from spleen of infected or uninfected WT mice were incubated for 18 h in RPMI 1640 medium with splenocyte supernatant from the same mouse. Il17a gene expression in purified neutrophil populations was examined by quantitative PCR. Il17a gene quantitie was normalized to the expression of the reference gene β-actin. Concentration of IL-17 in supernatant was detected by ELISA. (e) Cytokine production by splenocytes obtained from infected or uninfected WT mice. ND, not detected. (f) Il17a expression in purified bone marrow neutrophils incubated in RPMI 1640 medium with splenocyte supernatant from the same mouse plus no antibody (-) or neutralizing antibody (Ab) to IL-1β, TGF-β, IL-6 or IL-23 (above lanes). Actb (which encodes β-actin) serves as a loading control throughout. Data shown in (d–f) are the mean ± SEM. Data are representative of three independent experiments with similar results.