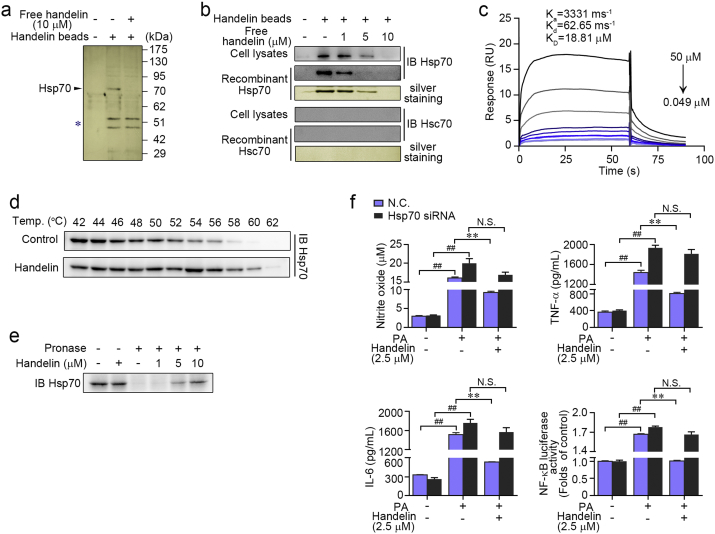
Fig. 2.
Identification of Hsp70 as a molecular target for handelin. (a) Handelin-conjugated sepharose beads were incubated with BV2 cells lysates in the presence or absence of excess handelin (10 μM). The intense ~ 70 kDa band was excised from the gel and identified as heat shock protein 70 (Hsp70). The asterisk marked bands indicated nonspecific beads-bound vimentin and actin. (b) Handelin-conjugated beads were incubated with cell lysates or a recombinant Hsp70 (or Hsc70) protein and handelin at concentrations shown, and then the proteins bound to the beads were detected by immunoblotting and silver staining. (c) Representative graph of surface plasmon resonance spectroscopy analysis showing the kinetics of increasing concentrations of handelin binding to human recombinant Hsp70. The kinetic parameters of Ka, Kd, and KD were derived by fitting to a 1:1 Langmuir binding model. (d) BV2 cells were exposed to handelin (10 μM) or vehicle followed by a cellular thermal shift assay. (e) BV2 cell lysates was incubated with handelin in the presence or absence of pronase (5 μg/mL). (f) Reverse effects of Hsp70 gene silence on handelin-mediated inhibition of nitrite oxide, TNF-α, IL-6 and NF-κB luciferase activity. Data are expressed as mean ± SEM for three individual experiments. ⁎P < 0.05, ⁎⁎P < 0.01 vs. PA group. ##P < 0.01 vs. control group. N.S., not significant.