Abstract
Background
Prior studies suggest that neuroblastomas that do not accumulate metaiodobenzyl-guanidine (MIBG) on diagnostic imaging (MIBG non-avid) may have more favorable features compared with MIBG avid tumors. We compared clinical features, biologic features, and clinical outcomes between patients with MIBG nonavid and MIBG avid neuroblastoma.
Procedure
Patients had metastatic high- or intermediate-risk neuroblastoma and were treated on Children’s Oncology Group protocols A3973 or A3961. Comparisons of clinical and biologic features according to MIBG avidity were made with chi-squared or Fisher exact tests. Event-free (EFS) and overall (OS) survival compared using log–rank tests and modeled using Cox models.
Results
Thirty of 343 patients (8.7%) had MIBG nonavid disease. Patients with nonavid tumors were less likely to have adrenal primary tumors (34.5 vs. 57.2%; P = 0.019), bone metastases (36.7 vs. 61.7%; P = 0.008), or positive urine catecholamines (66.7 vs. 91.0%; P < 0.001) compared with patients with MIBG avid tumors. Nonavid tumors were more likely to be MYCN amplified (53.8 vs. 32.6%; P =0.030) and had lower norepinephrine transporter expression. Patients with MIBG non-avid disease had a 5-year EFS of 50.0% compared with 38.7% for patients with MIBG avid disease (P = 0.028). On multivariate testing in high-risk patients, MIBG avidity was the sole adverse prognostic factor for EFS identified (hazard ratio 1.77; 95% confidence interval 1.04–2.99; P = 0.034).
Conclusions
Patients with MIBG nonavid neuroblastoma have lower rates of adrenal primary tumors, bone metastasis, and catecholamine secretion. Despite being more likely to have MYCN-amplified tumors, these patients have superior outcomes compared with patients with MIBG avid disease.
Keywords: avidity, MIBG, MYCN, norepinephrine transporter, neuroblastoma
1 INTRODUCTION
Neuroblastoma is a pediatric tumor derived from the sympathetic nervous system. Metaiodobenzylguanidine (MIBG) radiolabeled with 123I or 131I plays an important role in imaging and therapy in patients with neuroblastoma.1-3 MIBG uptake is mediated by the norepinephrine transporter (NET),4-6 though expression of other transporters has also been shown to correlate with MIBG uptake.7
Prior reports showed that some neuroblastomas do not accumulate MIBG, with estimates of approximately 10%.8-10 The extent to which patients with MIBG nonavid neuroblastoma differ from patients with MIBG avid disease is largely unknown. Previous smaller studies have suggested that these patients are more likely to have low-stage disease and less likely to secrete catecholamines, such as vanillylmandelic acid (VMA) and homovanillic acid (HVA).11,12 In addition, ganglioneuroma and ganglioneuroblastoma have been reported to be more likely to be MIBG nonavid compared with neuroblastoma histologies.11 In small case series, clinical outcomes for these patients appear to be favorable.11,12
Previous work by our group demonstrated superior event-free survival (EFS) for patients with MIBG nonavid tumors compared to patients with MIBG avid disease.10 That comparison focused exclusively on patients with high-risk disease and did not evaluate other potential differences between these groups. The goal of the current study therefore was to extend this analysis to obtain a more comprehensive understanding of patients with MIBG nonavid neuroblastoma, including both intermediate-risk and high-risk patients. We sought to compare clinical features, tumor biology, and clinical outcomes between patients with MIBG avid and nonavid disease.
2 METHODS
2.1 Patients
Patients were eligible for the overall analytical cohort if they met either of the following criteria. The high-risk group comprised 306 patients with metastatic high-risk neuroblastoma treated on Children’s Oncology Group (COG) protocol A397313 who had MIBG scans that were previously centrally reviewed as part of a prior analysis.10 The intermediate-risk cohort comprised 37 patients treated on COG protocol A396114 and who were coded as having metastatic disease and at least one bone metastasis. Since MIBG scans from A3961 were not available for central review, the latter criterion was included to reduce the likelihood of a false report of MIBG negativity in patients with locoregional disease who underwent tumor resection prior to baseline MIBG scan.
2.2 Dependent and independent variables
The primary dependent variable of interest was MIBG avidity based upon MIBG scan at initial diagnosis. For patients treated on protocol A3973, baseline MIBG scans were centrally reviewed10 and the outcome of that review (MIBG avid vs. MIBG nonavid) was used in the current analysis. For patients treated on protocol A3961, data on MIBG avidity were collected at the time of enrollment.
Key independent variables of interest were available from data collected as part of A3961, A3973, and the COG neuroblastoma biology study. Sex, age, primary site, metastatic sites, and secretion of urinary catecholamines (available only for A3973) were key clinical features. The presence of bone metastases was recorded at diagnosis based upon all available imaging data, with bone scan required at study entry on both A3961 and A3973 protocols. Biologic factors included MYCN status, ploidy, specific segmental chromosomal aberrations, histologic diagnosis, International Neuroblastoma Pathology Classification15 category, mitotic karyorrhectic index (MKI),16 and grade. EFS and overall survival (OS) rates were determined as described in the next section.
A subset of 23 patients (18 MIBG avid and 5 nonavid) in the analytical cohort were also included in the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) project and had gene expression data available from Affymetrix HuEx Arrays for secondary analysis for this project, with raw data processing and normalization as previously reported.17 For presented figures and statistical tests, gene expression data were log base 2 transformed from their raw values. We analyzed mRNA expression levels for genes encoding a panel of membrane transporters (NET, vesicular monoamine transporter 1 [VMAT1], VMAT2, somatostatin receptor 1 and 2, dopamine receptor D2, electroneutral sodium bicarbonate exchanger 1, monocarboxylate transporter 9, and sodium/potassium/calcium exchanger 5) and encoding enzymes involved in neurotransmitter metabolism (catechol-O-methyltransferase, dopamine beta-hydroxylase, glutamate decarboxylase 1, histidine decarboxylase, phenylethanolamine-N-methyltransferase, tryptophan hydroxylase 1, and tyrosine hydroxylase).
2.3 Statistical methods
Comparisons of the distribution of clinical and biologic features according to MIBG avidity were made using chi-square test, unless any cell in a contingency table contained less than five observations, in which case Fisher exact test was used. No correction for multiple testing was performed. EFS was determined using the Kaplan–Meier method18 as the time from enrollment to first episode of relapse, disease progression, death, or second malignancy, with patients without event censored at time of last follow-up. OS was determined using the Kaplan–Meier method as the time from enrollment to death, with surviving patients censored at the time of last follow-up. Differences in EFS and OS rates were assessed with log–rank tests. Cox proportional hazards regression models of EFS and OS were constructed using backward selection methods, with MIBG avidity retained as a fixed variable in all models. The proportional hazards assumption was confirmed by testing time-dependent covariates. These analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Gene expression data were compared using a series of Welch t-tests performed in R.
3 RESULTS
3.1 Incidence of MIBG nonavid disease
Among patients in the combined cohort of intermediate- and high-risk disease, 30/343 (8.7%) patients had MIBG nonavid disease. Among patients with intermediate-risk disease (protocol A3961), only 1/37 (2.7%) patient had MIBG nonavid disease. Among patients in the high-risk cohort (protocol A3973), 29/306 (9.5%) patients had MIBG non-avid disease.
3.2 Clinical and biologic features differ in MIBG avid versus nonavid neuroblastoma
We first evaluated clinical and biologic features in the pooled cohort of patients with intermediate-risk and high-risk neuroblastoma (Table 1). Patients with nonavid tumors were less likely to have adrenal primary tumors or bone metastases compared with patients with MIBG avid tumors. Nonavid tumors were more likely to be MYCN amplified. There was no difference in the proportion of patients with ganglioneuroblastoma between MIBG avid and nonavid groups.
TABLE 1.
Clinical and biologic features according to MIBG avidity
| Full cohort
|
High-risk cohort only
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | All patients (n = 343) | MIBG nonavid (n = 30) | MIBG avid (n = 313) | P value | All patients (n = 306) | MIBG nonavid (n = 29) | MIBG-avid (n = 277) | P value |
| Male | 195 | 20 (66.7%) | 175 (55.9%) | 0.256 | 182 | 19 (65.5%) | 163 (58.8%) | 0.486 |
| Female | 148 | 10 (33.3%) | 138 (44.1%) | 124 | 10 (34.5%) | 114 (41.2%) | ||
|
| ||||||||
| Age ≥18 months | 272 | 25 (83.3%) | 247 (78.9%) | 0.568 | 272 | 25 (86.2%) | 247 (89.2%) | 0.545a |
| Age <18 months | 71 | 5 (16.7%) | 66 (21.1%) | 34 | 4 (13.8%) | 30 (10.8%) | ||
|
| ||||||||
| Adrenal primary | 176 | 10 (34.5%) | 166 (57.2%) | 0.019 | 155 | 10 (35.7%) | 145 (56.6%) | 0.035 |
| Other primary sites | 143 | 19 (65.5%) | 124 (42.8%) | 129 | 18 (64.3%) | 111 (43.4%) | ||
|
| ||||||||
| Thoracic primary | 17 | 3 (10.3%) | 14 (4.8%) | 0.193a | 13 | 3 (10.7%) | 10 (3.9%) | 0.125a |
| Other primary sites | 302 | 26 (89.7%) | 276 (95.2%) | 271 | 25 (89.3%) | 246 (96.1%) | ||
|
| ||||||||
| Bone metastasis | 204 | 11 (36.7%) | 193 (61.7%) | 0.008 | 167 | 10 (34.5%) | 157 (56.7%) | 0.022 |
| No bone metastasis | 139 | 19 (63.3%) | 120 (38.3%) | 139 | 19 (65.5%) | 120 (43.3%) | ||
|
| ||||||||
| Bone marrow metastasis | 218 | 15 (50.0%) | 203 (64.9%) | 0.106 | 191 | 15 (51.7%) | 176 (63.5%) | 0.211 |
| No bone marrow metastasis | 125 | 15 (50.0%) | 110 (35.1%) | 115 | 14 (48.3%) | 101 (36.5%) | ||
|
| ||||||||
| MYCN amplified | 98 | 14 (53.8%) | 84 (32.6%) | 0.030 | 98 | 14 (56.0%) | 84 (37.8%) | 0.078 |
| MYCN nonamplified | 186 | 12 (46.2%) | 174 (67.4%) | 149 | 11 (44.0%) | 138 (62.2%) | ||
|
| ||||||||
| Diploid | 152 | 17 (65.4%) | 135 (53.6%) | 0.249 | 132 | 16 (64.0%) | 116 (53.7%) | 0.328 |
| Hyperdiploid | 126 | 9 (34.6%) | 117 (46.4%) | 109 | 9 (36.0%) | 100 (46.3%) | ||
|
| ||||||||
| LOH/aberration at 1p | 25 | 2 (33.3%) | 23 (39.7%) | 1.000a | 22 | 2 (33.3%) | 20 (43.5%) | 1.000a |
| No 1p LOH/aberration | 39 | 4 (66.7%) | 35 (60.3%) | 30 | 4 (66.7%) | 26 (56.5%) | ||
|
| ||||||||
| Aberration at 11q | 19 | 1 (20.0%) | 18 (35.3%) | 0.652a | 16 | 1 (20.0%) | 15 (35.7%) | 0.648a |
| No 11q aberration | 37 | 4 (80.0%) | 33 (64.7%) | 31 | 4 (80.0%) | 27 (64.3%) | ||
|
| ||||||||
| Neuroblastoma | 221 | 20 (80.0%) | 201 (81.0%) | 0.718a | 197 | 19 (79.2%) | 178 (79.5%) | 0.730a |
| Ganglioneuroblastoma | 22 | 3 (12.0%) | 19 (7.7%) | 22 | 3 (12.5%) | 19 (8.5%) | ||
| Other | 30 | 2 (8.0%) | 28 (11.3%) | 29 | 2 (8.3%) | 27 (12.0%) | ||
|
| ||||||||
| Unfavorable histology | 220 | 21 (91.3%) | 199 (83.3%) | 0.550a | 218 | 21 (95.5%) | 197 (96.6%) | 0.565a |
| Favorable histology | 42 | 2 (8.7%) | 40 (16.7%) | 8 | 1 (4.5%) | 7 (3.4%) | ||
|
| ||||||||
| High MKI | 70 | 11 (50.0%) | 59 (31.6%) | 0.083 | 69 | 11 (52.4%) | 58 (34.9%) | 0.119 |
| Low/Intermediate MKI | 139 | 11 (50.0%) | 128 (68.4%) | 118 | 10 (47.6%) | 108 (65.1%) | ||
|
| ||||||||
| Un-/poorly differentiated | 200 | 19 (86.4%) | 181 (91.0%) | 0.447a | 179 | 18 (85.7%) | 161 (90.4%) | 0.450a |
| Differentiating | 21 | 3 (13.6%) | 18 (9.0%) | 20 | 3 (14.3%) | 17 (9.6%) | ||
|
| ||||||||
| Catecholamine + | Not Evaluatedb | 260 | 18 (66.7%) | 242 (91.0%) | <0.001 | |||
| Catecholamine - | 33 | 9 (33.3%) | 24 (9.0%) | |||||
Fisher’s exact test.
Data not collected for intermediate-risk patients, so variable only analyzed for high-risk cohort.
We next repeated these analyses exclusively in patients with high-risk neuroblastoma, all of whom had stage 4 disease and centrally reviewed baseline MIBG scans (Table 1). Patients with nonavid tumors were less likely to have adrenal primary tumors, bone metastasis, or positive urine catecholamines.
3.3 MIBG avidity is an adverse prognostic factor in neuroblastoma
In the pooled cohort with intermediate-risk and high-risk disease, patients with MIBG avid disease had inferior clinical outcomes (Fig. 1). Patients with MIBG avid disease had a 5-year EFS of 38.7 ± 2.9% compared with 50.0 ± 9.5% for patients with MIBG nonavid disease (Fig. 1A; P = 0.028). Patients with MIBG avid disease had 5-year OS of 52.2 ± 2.9% compared with 66.5 ± 9.1% for patients with nonavid disease (Fig. 1B; P = 0.104). On multivariate testing, the hazard ratio for an event for patients with MIBG avid tumors was 1.77 (95% confidence interval 0.98–3.19; P = 0.059) after adjusting for significant variables (age and ploidy) following backward selection. MIBG avidity was not a significant predictor of OS on multivariate testing after adjusting for other significant variables (age, MYCN status, and MKI) following backward selection (hazard ratio for death for MIBG avid patients = 1.46 with 95% confidence interval 0.73–2.91; P = 0.283).
FIGURE 1.
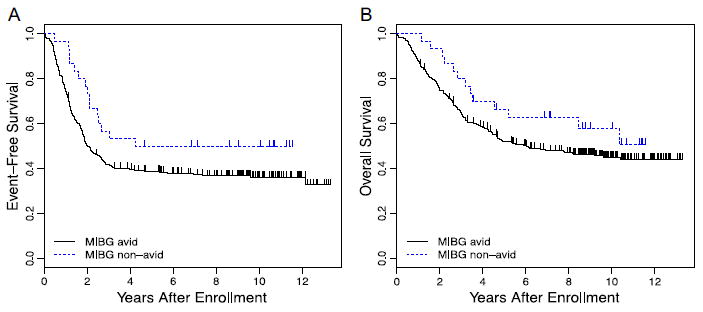
(A) Kaplan–Meier estimates of EFS for patients with intermediate- and high-risk neuroblastoma according to MIBG avidity (n = 313 avid and 30 nonavid; P = 0.028). (B) Kaplan–Meier estimates of OS for patients with intermediate- and high-risk neuroblastoma according to MIBG avidity (n = 313 avid and 30 non-avid; P = 0.104)
We next repeated these analyses exclusively in patients with high-risk neuroblastoma. In this cohort, MIBG avidity was an adverse prognostic factor for EFS and OS (Fig. 2). Patients with MIBG avid disease had a 5-year EFS of 33.5 ± 2.9% compared with 48.4 ± 9.6% for patients with MIBG nonavid disease (Fig. 2A; P = 0.012). Patients with MIBG avid disease had a 5-year OS of 47.3 ± 3.1% compared with 65.3 ± 9.3% for patients with MIBG nonavid disease (Fig. 2B; P = 0.049).
FIGURE 2.
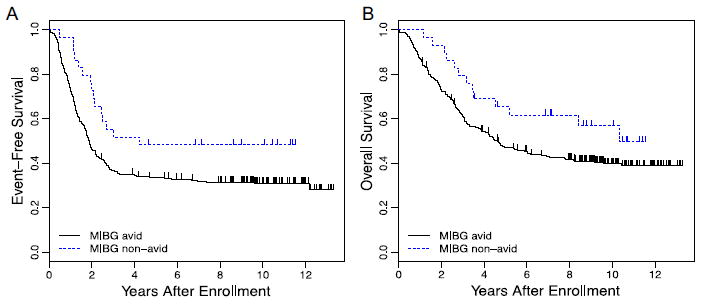
(A) Kaplan–Meier estimates of EFS for patients with high-risk neuroblastoma according to MIBG avidity (n =277 avid and 29 nonavid; P = 0.012). (B) Kaplan–Meier estimates of OS for patients with high-risk neuroblastoma according to MIBG avidity (n = 277 avid and 29 nonavid; P = 0.049)
On multivariate testing in high-risk patients, MIBG avidity was the sole adverse prognostic factor for EFS identified, while MYCN status, age, grade, MKI, and ploidy were not prognostic. Patients with MIBG avid disease had a hazard ratio for event of 1.77 (95% confidence interval 1.04–2.99; P = 0.034) compared with patients with MIBG nonavid disease. MIBG avidity was not a significant predictor of OS on multivariate testing after adjusting for other significant variables (MKI) following backward selection (hazard ratio for death for MIBG avid patients = 1.54 with 95% confidence interval 0.78–3.1; P =0.219).
3.4 Gene expression differs between MIBG avid versus nonavid neuroblastoma
Of the membrane transporters investigated, only expression for the gene encoding NET differed between MIBG avid versus. nonavid tumors (Fig. 3). The median NET mRNA expression was 9.1 (range 6.3–10.1) for MIBG avid tumors versus 7.6 (range 6.1–8.6) for MIBG non-avid tumors (P =0.052). Expression of genes encoding other membrane transporters, including VMAT2, did not differ between groups. Likewise, expression of genes encoding enzymes involved in neurotransmitter metabolism did not differ between groups.
FIGURE 3.
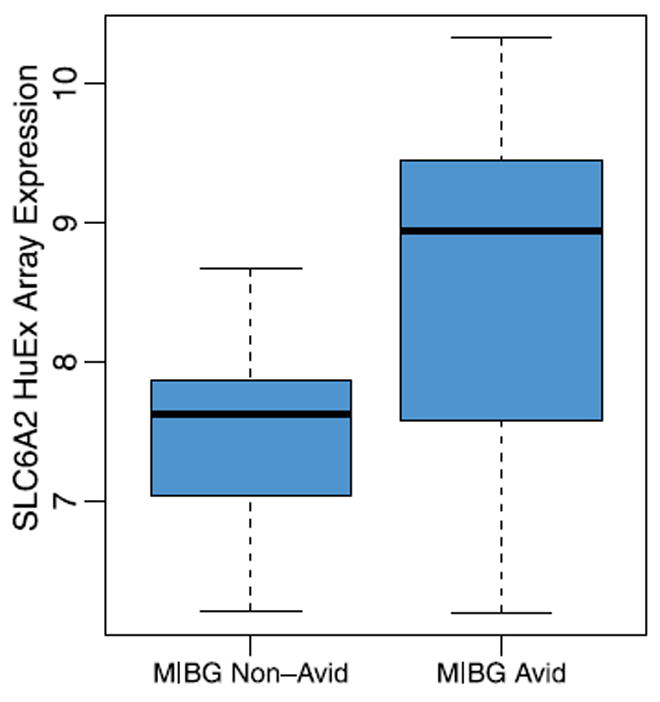
Distribution of NET mRNA expression between patients with MIBG avid (n =18) and nonavid (n =5) tumors. Data are presented as log base 2 values of normalized expression data
4 DISCUSSION
In this comprehensive analysis of MIBG nonavid neuroblastoma, we made several observations. We noted for the first time that MIBG non-avid tumors have higher rates of MYCN amplification, a finding that may reflect the extent of neural differentiation of these tumors. We also observed a unique pattern of sites of disease involvement for these tumors, with lower rates of adrenal primary tumors and of bone metastatic tumors. Our univariate and multivariate analyses also confirmed reports from smaller studies indicating superior outcomes in MIBG nonavid neuroblastoma.11,12
One of our most striking observations was the higher rate of MYCN amplification among patients with MIBG nonavid tumors, with the majority of nonavid tumors harboring MYCN amplification. Previous work has shown lower levels of NET and VMAT proteins in MYCN-amplified tumors.4,7 Taken together with our current findings, these observations suggest that MYCN amplification results in tumor cells that are less likely to express mature neural features, providing a mechanism for their lack of MIBG uptake. Another group has previously reported that, among MIBG avid tumors, MYCN status is not associated with intensity of MIBG uptake.19 However, MYCN-amplified tumors have been reported to lead to more focal uptake on MIBG scans, which may have increased the potential for false negative interpretation.20
We were able to leverage data from the TARGET initiative to determine whether expression of key genes of interest differ between MIBG avid and nonavid tumors. We demonstrated that NET mRNA expression, but not VMAT2 mRNA expression, is lower in MIBG nonavid tumors. This finding is contrary to prior work by our group that relied on polymerase chain reaction assays for NET mRNA expression and failed to demonstrate a difference in NET gene expression between MIBG avid versus nonavid tumors.4 This discrepancy may reflect differences in methodology and/or tumor material between studies. We noted lower rates of urinary catecholamine secretion in patients with MIBG nonavid tumors. These findings suggest that MIBG nonavid tumors may be characterized by general downregulation of neural features. However, similar levels of expression of genes involved in neurotransmitter metabolism between avid and nonavid tumors are not consistent with this hypothesis. Importantly, though, our sample size with available TARGET data may have limited our ability to detect differences.
MIBG avidity was associated with sites of primary and metastatic disease. Patients with MIBG nonavid tumors were significantly less likely to have adrenal tumors. The etiology for this novel observation is unclear. Previous work focused on evaluation of putative MIBG membrane transporters (NET and VMAT) did not show differential expression of these transporters between adrenal and nonadrenal tumors.4,7 Our findings also suggest a lower rate of bone metastasis among patients with MIBG nonavid disease. It is not clear if this finding represents a true reduction in bone involvement or an ascertainment issue given differential sensitivity between MIBG versus bone scans. While bone scans were required at study entry for both A3961 and A3973, the results of these scans are not available as part of the current analysis. We note that prior analyses from the International Neuroblastoma Risk Group reported higher rates of MYCN amplification among patients with adrenal primary tumors and among patients with bone metastasis.21,22 Given that the cohort of patients with MIBG non-avid disease was enriched for patients with MYCN-amplified disease, the lower rates of adrenal primary tumors and of bone metastasis in this group are unanticipated findings and suggest that these observations are not confounded by the association between MIBG avidity and MYCN status.
Our finding that patients with MIBG non-avid tumors have superior clinical outcomes confirms prior smaller studies that suggested this pattern.11,12 We extended those prior observations by noting that MIBG avidity was the sole adverse prognostic factor for EFS among patients with high-risk disease. This finding is perhaps even more striking given that the cohort of patients with MIBG nonavid tumors was enriched with patients with MYCN amplification, an important adverse prognostic factor.23 The mechanism by which MIBG avidity impacts survival is unknown and cannot be addressed by our available data. One hypothesis is that MIBG nonavid tumors show less neural differentiation and are therefore more sensitive to the cytotoxic agents that form the cornerstone of neuroblastoma therapy. Using conventional INPC grading, we were not able to detect a difference in grade between MIBG avid and nonavid tumors, as nearly all tumors were undifferentiated or poorly differentiated.
Our study benefited from a relatively large group of patients with MIBG nonavid disease, a rare subset of neuroblastoma. MIBG avidity status was centrally reviewed for the majority of patients and for all high-risk patients. The observation that MIBG nonavid patients have superior EFS was reported previously for the high-risk cohort.10 We extended that and prior observations by evaluating not just clinical outcomes but also baseline clinical and biologic factors. We also acknowledge some important limitations of our work. In order to be more confident in the accuracy of MIBG avidity status, we focused exclusively on patients with metastatic disease. The extent to which our findings will generalize to patients with low-risk, localized intermediate-risk, or localized high-risk disease is unknown. In addition, scans from patients with intermediate-risk disease were not available for central review. Finally, only a limited number of patients had data available from the TARGET initiative. Further study of biologic differences between MIBG avid and nonavid tumor cells and associated microenvironment is needed.
Our findings provide important new information about the complex interaction between MIBG avidity, MYCN amplification, and sites of disease involvement. In addition, these patients had superior outcomes in the context of this retrospective analysis, though outcomes remain unsatisfactory even for patients with MIBG nonavid disease. The COG plans to validate this finding prospectively as part of an upcoming phase 3 clinical trial for patients with newly diagnosed high-risk neuroblastoma. If validated, future studies may consider alternative treatment approaches that reflect the unique biology of these tumors. As new imaging tools to quantify extent of MIBG avidity become available, future studies may also explore differences based upon the extent of MIBG avidity among patients with MIBG avid disease.
Acknowledgments
Supported by NIH grants: U10-CA098543; U10-CA98413; U10-CA29139; U10-CA180899; U10-CA180886; RC1MD004418 to the TARGET consortium; and T32-HG000046. Additional support provided by Department of Defense Grant PR120935, Alex’s Lemonade Stand Foundation, and the Ben Towne Foundation. The funding sources did not play a role in study design, conduct, data analysis, interpretation, or manuscript preparation. The authors gratefully acknowledge the support of the staff at the Imaging and Radiation Oncology Core Group (IROC) Rhode Island for facilitating central review of MIBG scans.
Abbreviations
- COG
Children’s Oncology Group
- EFS
event-free survival
- HVA
homovanillic acid
- LOH
loss of heterozygosity
- MIBG
metaiodobenzylguanidine
- MKI
mitotic karyorrhectic index
- NET
norepinephrine transporter
- OS
overall survival
- VMA
vanillylmandelic acid
- VMAT
vesicular monoamine transporter
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no relevant disclaimers or disclosures.
References
- 1.DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol. 2008;35(Suppl 1):S35–S48. doi: 10.1016/j.nucmedbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taggart D, Dubois SG, Matthay KK. Radiolabeled metaiodobenzyl-guanidine for imaging and therapy of neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:403–418. [PubMed] [Google Scholar]
- 3.Wilson JS, Gains JE, Moroz V, et al. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50:801–815. doi: 10.1016/j.ejca.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 4.DuBois SG, Geier E, Batra V, et al. Evaluation of norepinephrine transporter expression and metaiodobenzylguanidine avidity in neuroblastoma: a report from the Children’s Oncology Group. Int J Mol Imaging. 2012;2012:250834. doi: 10.1155/2012/250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glowniak JV, Kilty JE, Amara SG, et al. Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med. 1993;34:1140–1146. [PubMed] [Google Scholar]
- 6.Mairs RJ, Livingstone A, Gaze MN, et al. Prediction of accumulation of 131I-labelled meta-iodobenzylguanidine in neuroblastoma cell lines by means of reverse transcription and polymerase chain reaction. Br J Cancer. 1994;70:97–101. doi: 10.1038/bjc.1994.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temple W, Mendelsohn L, Kim GE, et al. Vesicular monoamine transporter protein expression correlates with clinical features, tumor biology, and MIBG avidity in neuroblastoma: a report from the Children’s Oncology Group. Eur J Nucl Med Mol Imaging. 2016;43:474–481. doi: 10.1007/s00259-015-3179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlin S, Mairs RJ, McCluskey AG, et al. Development of a real-time polymerase chain reaction assay for prediction of the uptake of meta-[(131)I]iodobenzylguanidine by neuroblastoma tumors. Clin Cancer Res. 2003;9:3338–3344. [PubMed] [Google Scholar]
- 9.Treuner J, Feine U, Niethammer D, et al. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984;1:333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 10.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s Oncology Group. J Nucl Med. 2013;54:541–548. doi: 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biasotti S, Garaventa A, Villavecchia GP, et al. False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med Pediatr Oncol. 2000;35:153–155. doi: 10.1002/1096-911x(200008)35:2<153::aid-mpo18>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Chan GC, Leung YL, Shing MM, et al. Does a “false negative” MIBG scan predict a better outcome in neuroblastoma patients? Med Pediatr Oncol. 2001;37:155. doi: 10.1002/mpo.1190. [DOI] [PubMed] [Google Scholar]
- 13.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 16.Teshiba R, Kawano S, Wang LL, et al. Age-dependent prognostic effect by Mitosis–Karyorrhexis Index in neuroblastoma: a report from the Children’s Oncology Group. Pediatr Dev Pathol. 2014;17:441–449. doi: 10.2350/14-06-1505-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnepp RW, Khurana P, Attiyeh EF, et al. A LIN28B-RAN-AURKA signaling network promotes neuroblastoma tumorigenesis. Cancer Cell. 2015;28:599–609. doi: 10.1016/j.ccell.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Fendler WP, Melzer HI, Walz C, et al. High (1)(2)(3)I-MIBG uptake in neuroblastic tumours indicates unfavourable histopathology. Eur J Nucl Med Mol Imaging. 2013;40:1701–1710. doi: 10.1007/s00259-013-2491-y. [DOI] [PubMed] [Google Scholar]
- 20.Bleeker G, van Eck-Smit BL, Zwinderman KH, et al. MIBG scans in patients with stage 4 neuroblastoma reveal two metastatic patterns, one is associated with MYCN amplification and in MYCN-amplified tumours correlates with a better prognosis. Eur J Nucl Med Mol Imaging. 2015;42:222–230. doi: 10.1007/s00259-014-2909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson D, Vo KT, London WB, et al. Identification of patient subgroups with markedly disparate rates of MYCN amplification in neuroblastoma: a report from the International Neuroblastoma Risk Group project. Cancer. 2016;122:935–945. doi: 10.1002/cncr.29848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vo KT, Matthay KK, Neuhaus J, et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2014;32:3169–3176. doi: 10.1200/JCO.2014.56.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]


