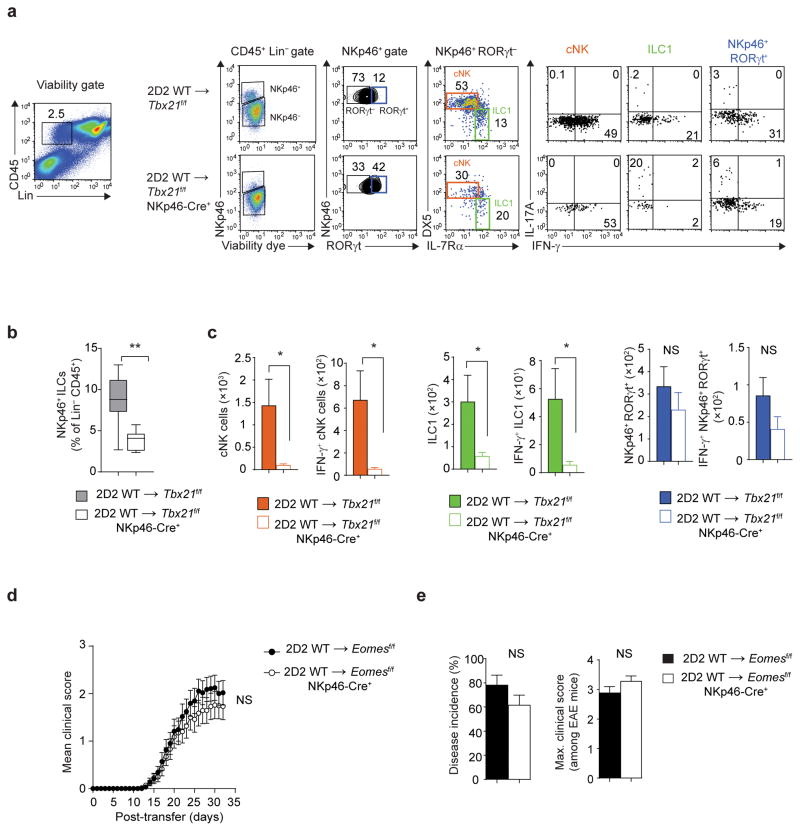
Figure 6.
Pathogenic function of T-bet maps to ILC1/NKp46+ ILC3. (a) Flow cytometry analysis of meningeal immune cells following adoptive transfer of 7.5 × 106 2D2 WT TH17 cells into Tbx21f/f or Tbx21f/f NKp46-Cre+ mice. Cells were stimulated with PMA and ionomycin for 3 h in the presence of monensin in the last 1.5 h of stimulation. Stimulated cells were stained with viability dye, antibodies to lineage markers (TCRβ, TCRγδ, B220, Gr1) and anti-CD45, anti-NKp46, anti-IL-7Rα, anti-CD49b (DX5), anti-RORγt, anti-IL-17A and anti-IFN-γ. Production of IL-17A and IFN-γ by meningeal NK cells (gated on Lin−CD45+NKp46+RORγt−DX5+IL-7Rα−), ILC1 (gated on Lin−CD45+NKp46+RORγt−DX5−/loIL-7Rα+) and NKp46+ ILC3 (gated on Lin−CD45+NKp46+RORγt+IL-7Rα+) was measured by intracellular cytokine staining. (b) The box-and-whiskers plot depict the frequency of NKp46+ ILCs within Lin−CD45+ population in the meninges of Tbx21f/f or Tbx21f/f NKp46-Cre+ mice. (c) Absolute numbers of NK cells, ILC1 and NKp46+ ILC3 and IFN-γ-producing NK cells, ILC1 and NKp46+ ILC3 (gated as in a) in the meninges of Tbx21f/f or Tbx21f/f NKp46-Cre+ mice. (d–e) Mean clinical scores, percent disease incidence and maximum clinical score of Eomesf/f or Eomesf/f NKp46-Cre+mice following adoptive transfer of 7.5 × 106 2D2 WT TH17 cells. NS, P > 0.05; *, P < 0.05; **, P < 0.001 (two-tailed Student’s t-test (b,c,e) or two-way ANOVA (d)). Data are combined from two independent experiments (b,c; n = 9, 10 mice per group) or three independent experiments (d,e; n = 29, 35 mice per group).