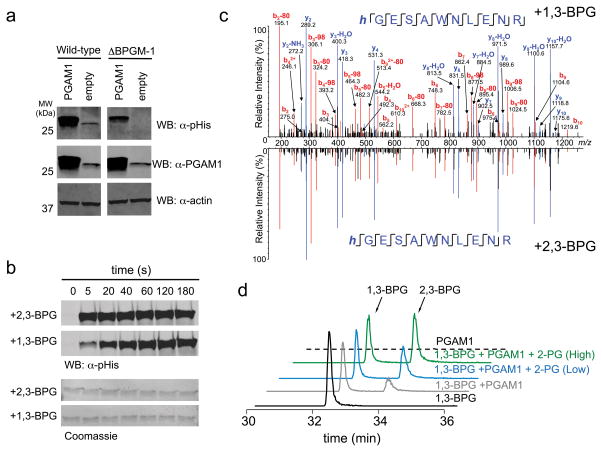
Figure 3. 1,3-BPG is an alternative source of PGAM1 phosphorylation.
a) Western blot analysis of PGAM1 overexpression lysates from wt and BPGM knockout HEK 293T cell lysates using an α–pHis and α–PGAM1 antibody (α–actin antibody was used as a loading control). b) Western blot analysis of a PGAM1 phosphorylation time course in the presence of 2,3-BPG or 1,3-BPG (membranes were stained with Coomassie to serve as a loading control). c) 1,3-BPG-treated PGAM1 was subjected to trypsinization and LC-MS analysis. Shown is the MS/MS spectrum of the tryptic peptide of PGAM1 containing the pHis site (His-11) and in mirror image is shown the MS/MS spectrum of 2,3-BPG induced phosphorylation of PGAM1 to demonstrate that both 1,3-BPG and 2,3-BPG result in His-11 phosphorylation. d) LC-MS analysis of PGAM1 phosphorylation with 1,3-BPG in the absence of 2-PG, low 2-PG (50 μM), or high 2-PG (1000 μM). Extracted ion chromatograms for 1,3-BPG and 2,3-BPG (m/z=264.952) are shown for each of the described reaction conditions. See Supplementary Figure 24 for full Western blot images.