Introduction
Myoepithelial cells possess dual immunohistochemical and ultrastructural features of smooth muscle cells and epithelial cells and are capable of divergent differentiation (1). As such, benign and malignant tumors of myoepithelial cells show a spectrum of morphologies and have been reported in a variety of anatomic locations, including but not limited to, the breast, larynx, soft tissues, salivary glands and more recently the skin (2–6). Benign mixed tumors, commonly identified in the skin (chondroid syringoma), salivary gland (pleomorphic adenoma) and less commonly in soft tissue also demonstrate myoepithelial differentiation, but differ from myoepitheliomas in that they have at least focal ductal differentiation (4). Cutaneous myoepithelioma is a recently described dermal neoplasm that is composed purely of myoepithelial cells and represents one end of a histological continuum of cutaneous myoepithelial neoplasms including chondroid syringoma and myoepithelial carcinoma (7, 8). Most cases of cutaneous myoepithelioma show a lobular or multinodular architecture of neoplastic myoepithelial cells with epithelioid, spindled, plasmacytoid or histiocytoid cytomorphology. However, a distinct subset of these neoplasms demonstrate a solid syncytial architecture and a slightly different immunohistochemical profile and may pose a diagnostic challenge (5, 6). This histologic variant has been termed cutaneous syncytial myopeithelioma (CSM)(6). Cutaneous myoepitheliomas of all morphologic types usually behave in a benign fashion, although there is some risk for local recurrence, especially if incompletely excised. Herein, we present two cases of CSM highlighting the unique immunohistochemical and molecular profile of this variant, which helps to differentiate it from other histological mimickers, particularly melanocytic lesions and epithelioid sarcoma, and the utility of a panel of immunostains and molecular studies in confirming the diagnosis.
Case presentation
Case 1
A 66 year old Caucasian female presented to her local dermatologist with a 4 month history of an enlarging red lesion on her right shoulder. Her past medical history was unremarkable. On physical examination, she was noted to have a solitary 0.7 × 0.7 cm erythematous firm papule on her right posterior deltoid. The rest of her physical examination including lymph node examination was within normal limits. The lesion was shaved and sent to the laboratory for histopathological examination. Upon initial review, the lesion was thought to represent an epithelioid cell histiocytoma, however, given its unusual features - specifically focal S100 positivity - the local dermatopathologist decided to send the case to our institution in consultation for further classification and to rule out the possibility of an atypical melanocytic proliferation.
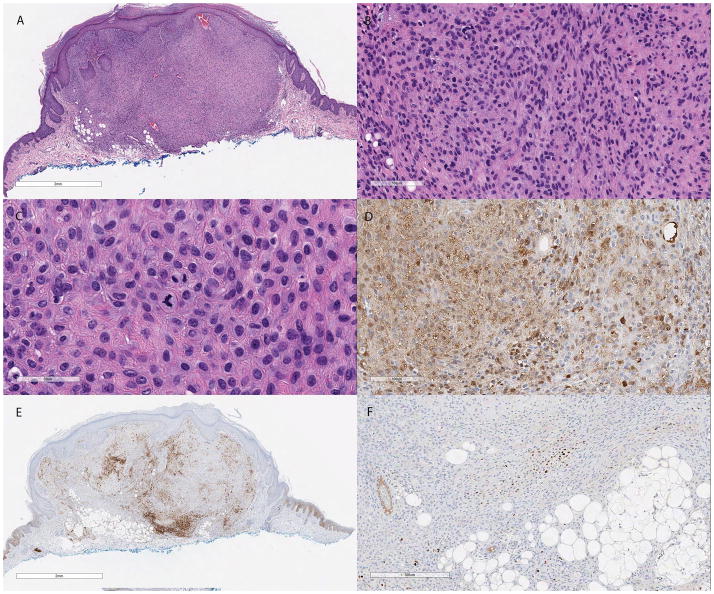
On histopathological examination, the lesion demonstrated a well-circumscribed proliferation of bland ovoid to spindle-shaped cells with a modest amount of pale eosinophilic cytoplasm, focally indistinct cell borders and a sheet-like growth pattern. Constituent nuclei were small with partially vesicular chromatin and small inconspicuous nucleoli. The cells showed minimal cytological atypia and mitoses were sparse, but present. Evidence of adipocytic metaplasia was noted at the base of the lesion and a mild inflammatory infiltrate composed of scattered lymphocytes and histiocytes was present focally at the periphery. The overlying epidermis was unremarkable, barring mild hyperplasia (Figure 1).
Figure 1.
(A) Low power H&E section showing a well circumscribed neoplasm with a syncytial growth pattern in the superficial dermis. Focal areas of adipocytic metaplasia are evident near the base of the lesion (H&E, 13×). (B) Constituent cells are bland, epithelioid and spindled with eosinophilic cytoplasm and monomorphic ovoid nuclei. Scant amounts of myxoid and collagenous stroma are also identified (H&E, 120×). (C) Rare mitotic figures were present in the superficial aspect of the lesion (H&E, 280×). (D) S100 immunohistochemical stain showing focal positivity (S100, 160×). (E) EMA (EMA, 13×) and (F) p63 are only focally positive (p63, 80×).
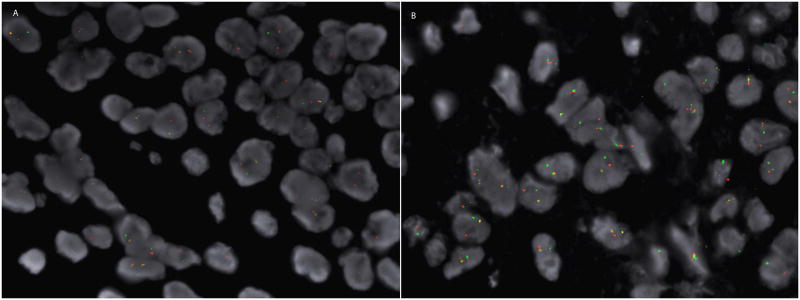
Immunohistochemical stains showed focal positive staining for S100, p63, epithelial membrane antigen (EMA), calponin, smooth muscle actin (SMA) and glial fibrillary acid protein (GFAP) (Figure 1). The cells were negative for MNF116 cytokeratin cocktail, high molecular weight keratin, melanocytic markers HMB-45 and MiTF and the histiocytic marker CD163. INI-1 nuclear staining was retained. A break-apart fluorescent in situ hybridization (FISH) probe showed rearrangement of the EWSR1 gene on chromosome 22q12 (Figure 3a).
Figure 3.
(A) The break apart FISH probe from case 1 shows separation of the red and green signals. (B) Similarly, constituent tumors cells from case 2 show separation of the red and green signals indicative of a rearrangement of the EWSR1 gene on chromosomal segment 22q12.
Based on these findings, a diagnosis of cutaneous syncytial myoepithelioma was rendered and conservative re-excision was recommended to clear the resection margins.
Case 2
A 38 year Caucasian female presented with a painful right lower leg “mole” that had been present for an unknown duration. Her past medical history was insignificant. On physical examination, she was found to have a 0.6 × 0.6 cm dome-shaped papule on her right lower leg. A shave biopsy was performed and the lesion was submitted for histopathological examination. Initial workup showed positive staining with pankeratin and the working differential diagnosis included epithelioid sarcoma with possible involvement of subcutaneous fat; however, given the challenging morphologic and immunohistochemical characteristics, it was sent to our institution for further characterization.
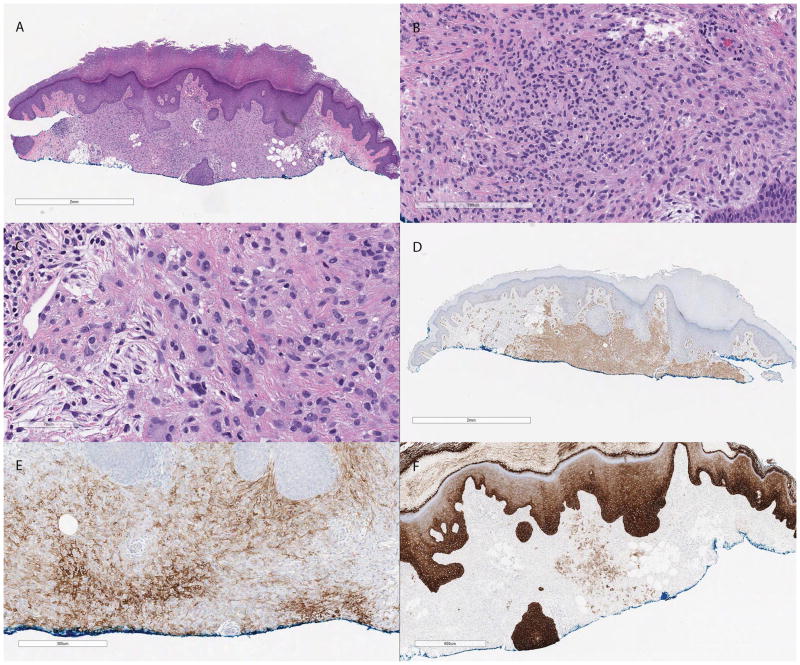
Histologically, the lesion was relatively well circumscribed and composed of bland spindled and epithelioid cells with focally indistinct cell borders present in a sheet-like growth pattern and scattered multinucleated cells. Nuclei were small with inconspicuous small nucleoli and no significant pleomorphism and the mitotic rate was very low. Focal adipocytic metaplasia and myxoid change were noted. Similar to the previous case, the overlying epidermis was unremarkable apart from mild hyperplasia (Figure 2).
Figure 2.
(A) Low power H&E section showing a partially sampled relatively well circumscribed proliferation in the superficial dermis with focal areas of adipocytic metaplasia (H&E, 13×). (B) Similar to the first case, this neoplasm is composed of epithelioid and spindled cells in a syncytial pattern showing minimal pleomorphism and scant amount of stroma (H&E, 120×). (C) Scattered multinucleated giant cells are present at the periphery of the lesion (H&E, 240×). (D) SMA is diffusely positive (SMA, 13×). (E) EMA (EMA, 100×) and (F) MNF116 are focally positive (MNF116, 30×).
Immunohistochemical stains performed on this case demonstrated diffuse immunoreactivity for CD56 and focal positivity for S100 (very focal), MNF116, EMA, SMA and caldesmon (Figure 2). Melanocytic markers, including Melan-A, MiTF, SOX10, and HMB-45 and histiocytic markers including CD68 and CD163 were all negative. In this case, p63 was negative. An EWSR1 gene rearrangement was also demonstrated in this case with a break-apart FISH probe (Figure 3).
Similar to the first cases, a diagnosis of cutaneous syncytial myoepithelioma was made and conservative re-excision was recommended to clear the resection margins.
Discussion
CSM is a distinctive variant of cutaneous myoepithelioma. It is characterized by a superficially located relatively well-circumscribed, unencapsulated, solid syncytial proliferation of cells with no evidence of ductal differentiation (6). Constituent cells have eosinophilic cytoplasm and demonstrate epithelioid or spindled cytomorphology. Nuclei are usually monomorphic, ovoid and lack significant hyperchromasia and pleomorphism. Low mitotic activity (up to 4 mitoses per 10 high-power fields) is commonly encountered (6, 9). In contrast to the more classic cases of cutaneous myoepithelioma, the syncytial variant has minimal associated stroma (5). In the current report however, both cases had focal scant, yet recognizable collagenous and myxoid stroma. Areas of adipocytic or chondro-osseous metaplasia are encountered in approximately one third of cases according to the original series published in 2013 (6). Recognition of this phenomenon is important to avoid the pitfall of diagnosing an infiltrative neoplasm with subcutaneous involvement, especially in partial biopsies, as was the case with our second patient.
Given the few reported cases of CSM, its true incidence is yet to be completely determined. Lack of familiarity with this myoepthelioma variant may pose diagnostic challenges for dermatopathologists. First, the hematoxylin and eosin (H&E) appearance and syncytial growth pattern usually generate a distinct differential diagnosis that differs from that of more classic cases of cutaneous myoepithelioma. Second, its immunohistochemical profile, with low incidence of keratin positivity and occasional weak staining for S100, may prevent one from recognizing myoepithelial differentiation in this tumor. Finally, molecular testing is critical for accurate diagnosis in most cases.
Epithelioid cell histiocytoma (ECH), also known as epithelioid benign fibrous histiocytoma, commonly enters the histological differential diagnosis of well-circumscribed bland dermal tumors with epithelioid morphology. Similar to CSM, it is characterized a by sheet-like growth of predominantly epithelioid cells with eosinophilic cytoplasm and scattered mitoses. Focal spindled cells and scattered multinucleated cells have also been reported. Relative to CSM, ECH has a more significant collagenous and vascular stromal component. Moreover, binucleate and multinucleate cells are more likely encountered in ECH (10, 11) In the largest report of CSM to date, the authors reported no binucleate or multinucleate cells in any of their 38 cases (6). In the current report, one case was found to have scattered multinucleate cells towards the periphery of the lesion. Whether these cells represent true tumor cells versus reactive multinucleated histiocytes remains unclear. Immunohistochemically, ECH shares with CSM positivity for EMA, however, it is consistently negative for S100 and other myoepithelial markers (10, 11). Another useful immunohistochemical marker is ALK, which has been recently reported to be positive (albeit focal in some cases) in nearly all ECH (12).
Other lesions that may enter the differential diagnosis include cellular neurothekeoma, juvenile xanthogranuloma and epithelioid perineurioma. Cellular neurothekeoma lacks the syncytial growth pattern and positive staining for S100, EMA and myoepithelial markers (13). Juvenile xanthogranuloma (JXG) may also enter the differential diagnosis, especially early JXG, where Touton giant cells are not as prominent (14). The unusual epithelioid variant of perineurioma shares a syncytial growth pattern of epithelioid cells and EMA positivity with CSM (15). The characteristic syncytial growth pattern of CSM, application of a battery of immunohistochemical stains to include keratin, S100 and other myoepithelial markers and EWSR1 FISH studies should allow distinction from the above entities in difficult cases.
Epithelioid sarcoma and atypical melanocytic proliferations such as intradermal Spitz nevi and nevoid melanomas represent the most clinically significant differential diagnoses. The presence of a diffuse proliferation of epithelioid cells expressing cytokeratin and/or EMA immunohistochemically may potentially lead to a misdiagnosis of epithelioid sarcoma. This is especially true when S100 expression is very focal and weak, as highlighted by our second case. Similarly, intradermal Spitz nevi and nevoid melanomas may show a relatively well circumscribed proliferation of epithelioid cells that are S100 positive, leading to a potential misdiagnosis. This pitfall is highlighted by our first case, where initial workup showed that the lesion was largely negative for cytokeratin cocktail. The lack of expression of other melanocytic markers in these situations, should prompt further workup to accurately distinguish a melanocytic lesion or nerve sheath tumor from a myoepithelial neoplasm, such as CSM. Again, a panel of carefully selected immunostains and molecular studies are indispensable when dermatopathologists are confronted with such scenarios.
The immunohistochemical profile of CSM is characteristic, although some variability does exist between cases. The most consistent marker is EMA, which shows a diffuse pattern in most reported cases (6). Occasionally, EMA is only focally positive, similar to our two cases (9). This could be explained by varying staining protocols between different laboratories or by reporting bias, where cases with focal staining are more likely to represent a diagnostic challenge and are more likely to be reported. All reported cases show at least focal staining for S100. However, as highlighted in our second case, it is important to emphasize that this focal staining can occasionally be very weak and hard to appreciate at low power, or in a partial biopsy. Other myopeithelial markers including SMA, GFAP, calponin, caldesmon and p63 are variably positive. Histiocytic and melanocytic markers (other than S100) are consistently negative. Relative to classic cases of cutaneous myoepithelioma, the incidence of keratin positivity is much lower in cases of CSM, with staining in only 14% of cases in a focal and weak pattern in the largest series of 38 cases (6). Only one of our cases showed a few scattered MNF116 positive cells.
Sox-10 has recently been reported to be positive in some cases of cutaneous myoepithelioma (3 of 5 cases, 60%), however, it appears that these cases were of the classic variety rather than the syncytial variant of cutaneous myoepithelioma (16). Although no definitive conclusions could be drawn, in one of our cases where SOX-10 was performed, constituent cells were negative.
In cases of CSM with weak expression of diagnostic myoepithelial markers, molecular testing is of paramount importance for diagnosis. In their series of 38 cases, Jo et al demonstrated EWSR1 gene rearrangements in 14 out of 16 cases (87.5%) of CSM, although a definitive fusion gene partner could not be demonstrated (6). In contrast, it seems that classic cutaneous myoepithelioma, which usually has a more consistent staining pattern, especially with keratins, is much less likely to harbor a EWSR1 gene rearrangement. This molecular aberration has been demonstrated in 2 of 7 cases in one series (17) and 2 of 8 cases in another (18).
In almost all reported cases, cutaneous myoepithelioma, including the syncytial variant, followed a benign course after complete resection. Few cases of local recurrence following incomplete excision have been reported (5, 6). Cases with significant mitotic activity and cellular pleomorphism have been designated as myoepithelial carcinomas, although definitive diagnostic criteria have not been elucidated for cutaneous lesions, in contrast to their soft tissue counterparts (5, 19). Despite their designation as carcinoma, these cases have low metastatic potential. In a recently published review, the authors found that 2 cases of cutaneous myoepithelial carcinoma recurred and 2 had lymph node and distant metastasis out of 7 cases with available follow up (20). However, it is important to note that some of these cases may have represented soft tissue myoepithelioma with secondary cutaneous involvement, including the authors’ own case.
In summary, CSM poses a diagnostic dilemma, even to experienced dermatopathologists. Its rarity, nonspecific clinical presentation, only somewhat distinct H&E features and variable immunophenotype add to its diagnostic difficulty. In these instances, seeking an expert opinion and performing molecular testing may be of significant value to rendering an accurate diagnosis of CSM, allowing distinction from potentially dangerous morphologic mimics, such as nevoid melanoma or epithelioid sarcoma.
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health (Bethesda, MD, SARC Sarcoma S.P.O.R.E. grant U54 CA168512).
References
- 1.Morinaga S, Nakajima T, Shimosato Y. Normal and neoplastic myoepithelial cells in salivary glands: an immunohistochemical study. Hum Pathol. 1987;18(12):1218. doi: 10.1016/s0046-8177(87)80404-5. [DOI] [PubMed] [Google Scholar]
- 2.Bigotti G, Di Giorgio CG. Myoepithelioma of the breast: histologic, immunologic, and electromicroscopic appearance. J Surg Oncol. 1986;32(1):58. doi: 10.1002/jso.2930320116. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Madrigal F, Santiago Payan H, Meneses A, Dominguez Malagon H, Rojas ME. Plasmacytoid myoepithelioma of the laryngeal region: a case report. Hum Pathol. 1995;26(7):802. doi: 10.1016/0046-8177(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick SE, Hitchcock MG, Kraus MD, Calonje E, Fletcher CD. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21(1):13. doi: 10.1097/00000478-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35(1):14. doi: 10.1016/j.humpath.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Jo VY, Antonescu CR, Zhang L, Dal Cin P, Hornick JL, Fletcher CD. Cutaneous syncytial myoepithelioma: clinicopathologic characterization in a series of 38 cases. Am J Surg Pathol. 2013;37(5):710. doi: 10.1097/PAS.0b013e3182772bba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutzner H, Mentzel T, Kaddu S, Soares LM, Sangueza OP, Requena L. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25(3):348. doi: 10.1097/00000478-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Mentzel T, Requena L, Kaddu S, Soares de Aleida LM, Sangueza OP, Kutzner H. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30(5):294. doi: 10.1034/j.1600-0560.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Huang HY, Lan J, Hwang CC, Liu CY. Cutaneous syncytial myoepithelioma: A case report with emphasis on the differential diagnosis of problematic dermal tumors. Oncol Lett. 2015;9(5):2275. doi: 10.3892/ol.2015.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle LA, Fletcher CD. EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol. 2011;38(9):697. doi: 10.1111/j.1600-0560.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 11.Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21(1):1. doi: 10.1097/00000372-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Doyle LA, Marino-Enriquez A, Fletcher CD, Hornick JL. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28(7):904. doi: 10.1038/modpathol.2015.49. [DOI] [PubMed] [Google Scholar]
- 13.Stratton J, Billings SD. Cellular neurothekeoma: analysis of 37 cases emphasizing atypical histologic features. Mod Pathol. 2014;27(5):701. doi: 10.1038/modpathol.2013.190. [DOI] [PubMed] [Google Scholar]
- 14.Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29(1):21. doi: 10.1097/01.pas.0000147395.01229.06. [DOI] [PubMed] [Google Scholar]
- 15.Haider SA, Lemberger RJ, Fisher C, McCulloch TA. Epithelioid perineurioma: an unusual variant. J Clin Pathol. 2008;61(10):1130. doi: 10.1136/jcp.2007.052092. [DOI] [PubMed] [Google Scholar]
- 16.Naujokas A, Charli-Joseph Y, Ruben BS, et al. SOX-10 expression in cutaneous myoepitheliomas and mixed tumors. J Cutan Pathol. 2014;41(4):353. doi: 10.1111/cup.12279. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49(12):1114. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flucke U, Palmedo G, Blankenhorn N, Slootweg PJ, Kutzner H, Mentzel T. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24(11):1444. doi: 10.1038/modpathol.2011.108. [DOI] [PubMed] [Google Scholar]
- 19.Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27(9):1183. doi: 10.1097/00000478-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Yokose C, Asai J, Kan S, et al. Myoepithelial carcinoma on the right shoulder: Case report with published work review. J Dermatol. 2016;43(9):1083. doi: 10.1111/1346-8138.13358. [DOI] [PubMed] [Google Scholar]