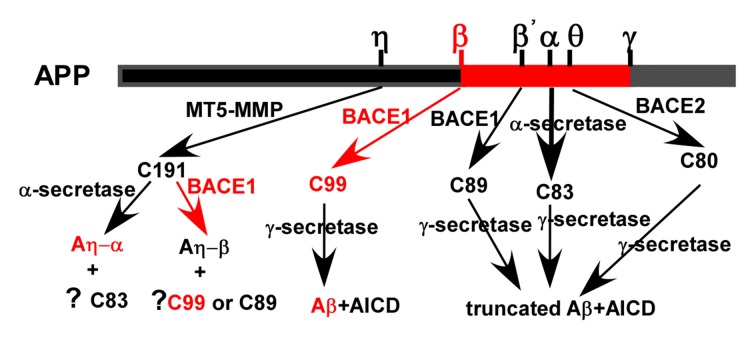
Figure 1.
Amyloid precursor protein (APP) processing and amyloid β (Aβ) generation. APP is mainly cleaved by α-secretase to generate secreted APP (sAPPα) and C-terminal fragment (CTF) of 83 amino acids (C83). C83 is further cleaved by γ-secretases to generates a truncated Aβ and APP intracellular domain (AICD), respectively. The minority of APP is cleaved by beta-site APP cleaving enzyme 1 (BACE1; β-secretase) at Asp1 and Glu11 (numbering for Aβ) sites to generate a CTF with 99 and 89 amino acids (C99 and C89), respectively. They were further cleaved by γ-secretase to produce Aβ and a truncated form of Aβ, respectively. APP is proteolyzed by BACE2 (θ-secretase) to generate a CTF with 80 amino acids (C80), which is further cleaved by γ-secretase to produce a truncated form of Aβ. Membrane type 5 matrix metalloproteinase (MT5-MMP; η-secretase) is revealed to cleave APP generating C191, which is further cleaved by α-secretase and β-secretase to produce Aη-α and Aη-β, respectively. However, the generation of C83, C99, C89 and their downstream cleavage products following η- cleavage remains elusive.