Table 1. The catalytic asymmetric cyclopropanation of styrene with FDA a .
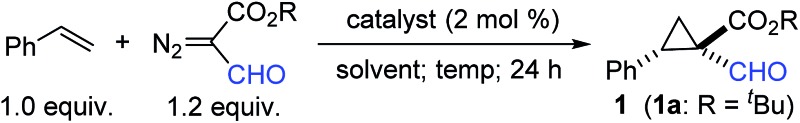
| |||||||
| Entry | Catalyst | R | Solvent | Temp. (°C) | Yield b (%) | (E) : (Z) | ee c (%) |
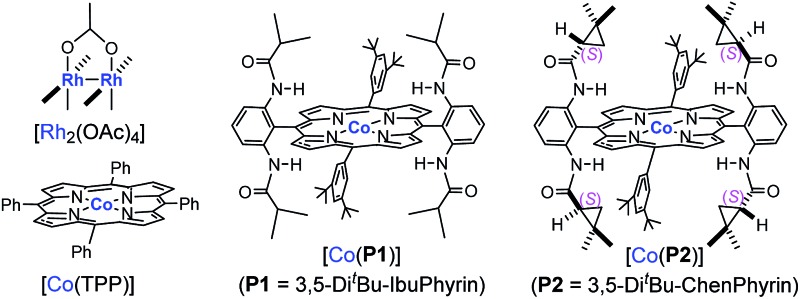
| 1 | [Rh2(OAc)4] | Et | DCM | 60 | 0 | — | — |
| 2 | [Co(TPP)] | Et | DCM | 60 | <10 d | — | — |
| 3 | [Co(P1)] | Et | DCM | 60 | 46 | 84 : 16 | — |
| 4 | [Co(P2)] | Et | DCM | 60 | 81 | 80 : 20 | 81 |
| 5 | [Co(P2)] | Et | EtOAc | 60 | 81 | 80 : 20 | 81 |
| 6 | [Co(P2)] | Et | PhCl | 60 | 73 | 82 : 18 | 83 |
| 7 | [Co(P2)] | Et | Hexanes | 60 | 73 | 80 : 20 | 82 |
| 8 | [Co(P2)] | Et | Toluene | 60 | 86 | 85 : 15 | 86 |
| 9 | [Co(P2)] | Et | Toluene | 40 | 73 | 86 : 14 | 90 |
| 10 | [Co(P2)] | Et | Toluene | RT | 60 | 87 : 13 | 93 |
| 11 | [Co(P2)] | t Bu | Toluene | 40 | 78 | 95 : 5 | 96 |
| 12 e | [Co( P2 )] | t Bu | Toluene | 40 | 84 | 95 : 5 | 96 |
| |||||||
aCarried out in one-portion under N2 with [olefin] = 0.20 M.
bIsolated yields.
cee of major (E)-diastereomer determined by chiral HPLC.
dDetermined by 1H-NMR.
eWith 5 mol% of catalyst for 20 h.
