Extended Data Figure 2. Phase separation is an isoform specific capability of phosphorylated HP1αthat is perturbed by GFP fusions.
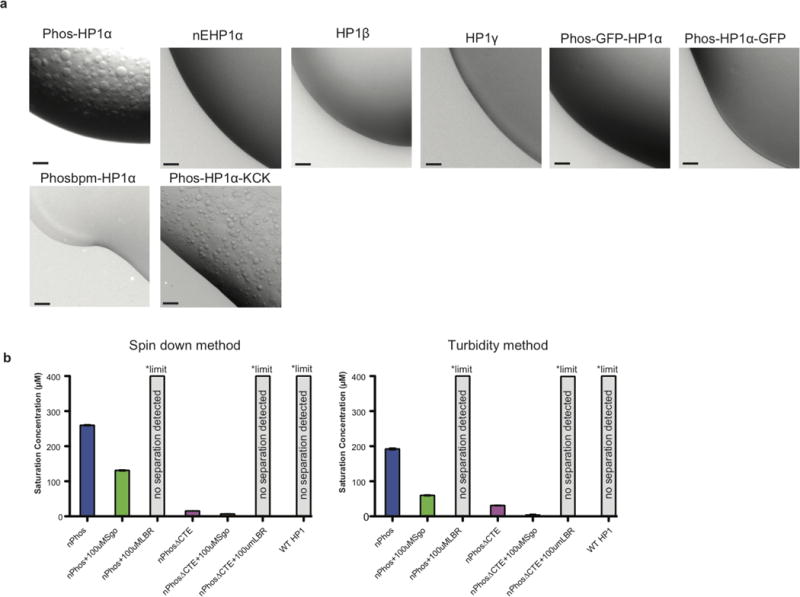
a, 1 μL of a solution of 400 μM of each protein was spotted on a plastic coverslip and imaged at 10×, scale bars are 50 μm. Buffer was 75 mM KCl, 20 mM HEPES pH 7.2, 1 mM DTT. Phos-HP1α is phosphorylated in the N-terminus and hinge, nE-HP1α has the N-terminal serines replaced with glutamates, Phos GFP-HP1α is a N-terminal GFP fusion phosphorylated in the N-terminus and hinge, Phos-HP1α-GFP is a C-terminal GFP fusion phosphorylated in the N-terminus and hinge, Phosbpm-HP1α has the ‘KRK’ hinge sequence mutated to alanines and phosphorylated in the N-terminus and hinge, Phos-HP1α-KCK has a C-terminal ‘GSKCK’ tag added and phosphorylated in the N-terminus and hinge. b, Complete comparison of saturation concentration measurements between spin down assay (left) and 340nm turbidity based measurement (right), some data is repeated from figure 1.
