Abstract
Objectives
On June 17, 2016, RESIST-TB, IMPAACT, Vital Strategies, and New Ventures jointly hosted the Pediatric Multidrug Resistant Tuberculosis Clinical Trials Landscape Meeting in Arlington, VA. The meeting provided updates on current multidrug-resistant tuberculosis (MDR-TB) trials targeting pediatric populations and adult trials that included pediatric patients.
Methods
A series of presentations were given that discussed site capacity needs, community engagement, and additional interventions necessary for clinical trials to improve the treatment of pediatric MDR-TB. This article presents a summary of topics discussed, including: current trials ongoing and planned; the global burden of MDR-TB in children; current regimens for MDR-TB treatment in children; pharmacokinetics of second-line anti-tuberculosis medications in children; design, sample size, and statistical considerations for MDR-TB trials in children; selection of study population, design, and treatment arms for a trial of novel pediatric MDR-TB regimens; practical aspects of pediatric MDR-TB treatment trials; and strategies for integrating children into adult tuberculosis trials.
Results
These discussions elucidated barriers to pediatric MDR-TB clinical trials and provided insight into necessary next steps for progress in this field.
Conclusions
Investigators and funding agencies need to respond to these recommendations so that important studies can be implemented, leading to improved treatment for children with MDR-TB.
Keywords: Tuerculosis, Multidrug Resistant Tuberculosis, MDR Tuberculosis, MDR-TB, Pediatric Multidrug Resistant Tuberculosis Clinical Tirals
Introduction
The global epidemic of multidrug-resistant (MDR) tuberculosis (TB; i.e. Mycobacterium tuberculosis that is resistant to isoniazid and rifampin) is a major threat to human health.1 In the past decade, there have been substantial improvements in our ability to diagnose and treat MDR-TB, however efforts have mainly focused on MDR-TB in adults. MDR-TB also has a substantial impact in children; currently, most MDR-TB treatment (and drug-susceptible TB) guidelines for children are extrapolated from adult data and rely on clinical experience instead of controlled trials. However differences in the pathophysiology, diagnosis, and treatment of childhood TB relative to TB in adults are well described, and have limited the benefit children have from recent advances in adult MDR-TB care.2 There are relatively few trials that focus specifically on considerations relevant to childhood TB. In order to address this deficit and begin the process of developing a science-based framework on which to base recommendations, the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) and Research Excellence to Stop TB Resistance (RESIST-TB) networks organized a meeting to bring together investigators and clinicians working in this field to summarize the current status of knowledge, identify important areas of research and develop plans for future research for pediatric MDR-TB. This report summarizes the results of this meeting, the “Pediatric MDR-TB Landscape Meeting”, held June 17, 2016, in Washington DC.
Update on current pediatric MDR-TB studies in progress
Prior to 2008, no clinical trials of MDR-TB treatment had ever been performed in adults or children, and treatment was based entirely on clinical opinion.3 Since then, Phase 2 clinical trials have demonstrated the efficacy of three new anti-TB drugs, bedaquiline, delamanid and pretomanid, for the treatment of MDR- and extensively drug-resistant (XDR)-TB.4 Moreover, linezolid, clofazimine and meropenem (existing drugs not previously used for MDR-TB) have been recognized to have activity against M. tuberculosis and therefore to be potential companion agents in new regimens for MDR-TB treatment.4 This has led to a long-overdue resurgence in MDR-TB treatment trials. By 2016, four phase 2 and one phase 3 MDR-TB treatment trials had been completed,5,6,7,8,9 and an additional eight phase 2 and eight phase 3 trials were under way.10 While this represents a welcome increase in activity that will hopefully expand treatment options for MDR-TB, only two of these trials are enrolling children under the age of 12, while one is enrolling adolescents age 13–17. Thus, there is a substantial unmet need for data that will guide the treatment of children with MDR-TB. This meeting reviewed clinical trials and observational cohort studies of pediatric MDR-TB in order to identify knowledge gaps and generate momentum for new studies to address those gaps.
Planned and ongoing pediatric MDR-TB studies can be divided into two groups: first, treatment studies in which the pharmacokinetics (PK) and safety in children with MDR-TB disease are characterized with the goal being to define optimal doses for children with TB disease, taking into account efficacy-toxicity tradeoffs (shown in Table 1), and second, studies of prophylactic therapies, in which pediatric household contacts of adult MDR-TB patients are treated with the goal of prevention of disease (shown in Table 2).
Table 1.
PK/safety studies in children with MDR-TB disease
| Study | Intervention | Design | Target Population | Aim | Sample Size |
|---|---|---|---|---|---|
| MDR-PK 1 | PK and safety of levofloxacin, moxifloxacin, ofloxacin, amikacin, high-dose isoniazid, ethionamide, Para-aminosalicylic acid, terizidone, cycloserine at routine doses | Observational cohort | Children 0–15 years old | PK, drug-drug interactions and safety in children treated for MDR-TB with/without HIV co-infection. | 318 |
| MDR-PK 2 | Moxifloxacin, levofloxacin and linezolid | Observational cohort | Children 0–15 years old | PK, drug-drug interactions and safety in children treated for MDR-TB with/without HIV co-infection. | 100 |
| Jansen C211 | Bedaquiline in combination with other second-line agents | Open label, single arm | HIV-uninfected children ages 0–1 years old; 2–4 years; 5–11 years; 12–17 years | PK, safety and anti-mycobacterial activity of bedaquiline in combination with other second line drugs | 60 |
| IMPAACT P1108 | Bedaquiline in combination with other second-line agents | Open label, single arm | HIV-infected and uninfected children with MDR-TB under an FDA IND | Dose finding and safety study of bedaquiline in combination with other second line drugs | Not yet enrolling |
| Otsuka 232 and 233 | Delamanid | Open label, multiple-dose | HIV-uninfected children 0–17 years old | PK, safety and tolerability of delamanid | 36 |
| IMPAACT 2005 | All-oral, injectable-sparing, delamanid-based MDR-TB regimen | Open label, single-arm clinical trial | Children 0 – <3 years; 3 – <6 years; 6 – <12 years; 12 – <18 years | PK, safety and tolerability of intervention regimen; assess effect of HIV and ART on delamanid PK | Not yet enrolling |
Table 2.
Trials of MDR-TB preventive therapy in children
| Study | Intervention | Design | Target Population | Aim | Sample Size |
|---|---|---|---|---|---|
| V-QUIN | Levofloxacin, 6 months | Randomized, double-blind, placebo-controlled phase 3 trial | Adult, child, and adolescent household contacts of pulmonary MDR-TB patients | Evaluate intervention compared to placebo for prevention of MDR-TB in household contacts | Not yet enrolling children |
| TB-CHAMP | Levofloxacin, 6 months | Randomized, double-blind, placebo-controlled phase 3 trial | Children <5 years old who are household contacts of pulmonary MDR-TB patients | Evaluate efficacy and safety of levofloxacin compared to placebo for prevention of MDR-TB | Not yet enrolling |
| A5300/P2003 | Delamanid | Open label, phase 3 trial | Adult and child (0–17 years) household contacts of MDR-TB patients | Evaluate efficacy and safety of delamanid compared to standard-dose isoniazid for TB prevention | Not yet enrolling |
Missing from these tables are any studies specifically evaluating the efficacy of novel regimens for treatment of pediatric MDR-TB disease, as none are ongoing. It is hoped that better understanding of the PK and safety of new and existing drugs will lead to the rational design of trials to evaluate optimized regimens specifically tailored to pediatric patients.
Global burden of MDR-TB in children
TB remains substantially under-diagnosed among children due to challenges with microbiologic confirmation,11 a dearth of good diagnostics, and limitations in the recording and reporting of pediatric TB.12 These challenges are further exacerbated in children with MDR-TB. Up until 2012, the World Health Organization did not provide estimates of the burden of pediatric TB. Two recent studies have provided evidence that the proportion of children with TB whose infecting strains are MDR reflects the proportion of new (i.e. never previously treated for TB) adult TB cases with MDR-TB in the same setting.13,14 The first estimate of pediatric MDR-TB incidence, published in 2014 by Jenkins et al., was 32,000 annual incident cases (3.2% of their TB incidence estimate).14 In 2016, Dodd et al. published an extension of their mathematical model to estimate the number of children with several different forms of drug-resistant TB.15 They estimated that 24,800 children developed MDR-TB annually (i.e. 2.9% of incident TB cases).
We also do not know what proportion of children with MDR-TB disease are diagnosed and what proportion of those children receive appropriate treatment. However, it is a very small proportion of the 25,000 – 32,000 children that develop MDR-TB annually. Despite the fact that children who are diagnosed and receive treatment for MDR-TB are likely to recover and have good treatment outcomes,16 those that remain undiagnosed have a high risk of death. A recent review of literature from the pre-treatment era demonstrated the high mortality in children who do not receive treatment for TB and given the high number of children with MDR-TB that are left untreated, mortality is likely to be significant.17
Regimens for MDR-TB treatment in children: preclinical-clinical translation?
To assess whether or not preclinical models can help inform clinical assessments of anti-TB drugs for children, we must first understand the characteristics of TB disease in children. Pediatric and adult TB are very different. The clinical manifestations of pediatric TB are highly variable and roughly correlate with age; very young children more commonly develop disseminated disease than older children and adults, and children aged 2–12 commonly have paucibacillary, non-cavitary disease limited to lung or lymph nodes, without caseous necrosis (see figure). Children over the age of 12 can present with adult-like pulmonary disease, often with lung cavitation and high bacterial burden.2 Since younger children tend to have paucibacillary TB (approximately 30% culture-confirmed and <10% sputum smear positive) they can reasonably be expected to respond to treatment better than adults. Improved treatment outcomes amongst children with MDR-TB compared to adults are already achieved despite substantially lower drug exposures in children for many key second-line drugs. However, this variability in disease severity, pathology, and mycobacterial burden (104 in paucibacillary versus 107 to 109 in cavitary disease)18 presents a challenge for selection of a single regimen and treatment duration to test for “pediatric MDR-TB.”
Figure 1.
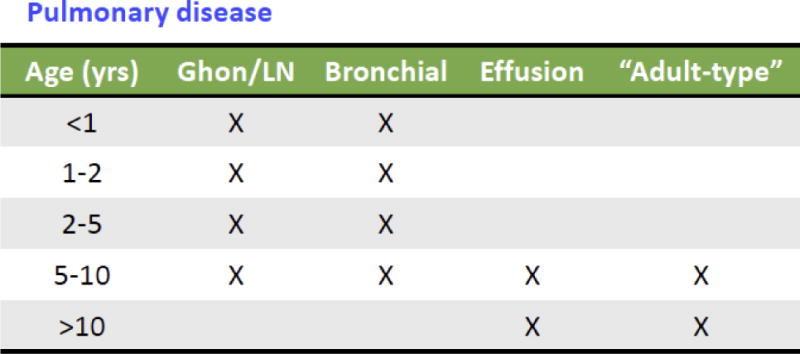
Manifestations of Pediatric tuberculosis, by age, adapted from Marais2
A critical concern for successful TB treatment is delivery of effective drugs at adequate concentrations to the site of disease. Penetration of TB drugs into macrophages, the central nervous system, lymph nodes, lung parenchyma, and cavitary contents may be needed for treatment of pediatric MDR-TB, depending on the age of the child and his or her associated TB-related pathology. Penetration coefficients of drugs into these different compartments vary widely.19 Studies assessing the spatial distribution of anti-TB drugs in relevant preclinical models may help inform selection of drugs and/or drug combinations for further testing in specific populations, e.g. children with disseminated, intracellular disease or lymphadenitis or meningitis. Drugs also differ in their ability to protect each other against the emergence of resistance. In patients with a high bacillary load, chromosomally-mediated resistance is invariably present in a subpopulation of organisms, so drugs must be given in combination to prevent emergence of these pre-existing resistant strains. So for adolescents with cavitary disease, it is likely that drugs must both penetrate into cavitary contents and achieve concentrations sufficient to protect companion drugs against emergence of resistance in that compartment. For children with paucibacillary disease, the number of drugs that is needed in a regimen to prevent acceleration of resistance is unknown but may be fewer.
There is no single best animal model for pediatric TB disease. In the “standard” mouse TB treatment model in balbC mice, the disease is largely intracellular, and the mice do not typically develop caseous necrosis or cavities, and thus their pathology is similar to that seen in young children.20 Animal models that develop necrotic lesions and/or cavitary disease (e.g. so-called Kramnik (C3HeB/FeJ) mice or select rabbit models) may be more akin to, and informative about, adolescent TB disease. Thus, no single animal model has been validated as a pediatric TB treatment model. Indeed, given the wide spectrum of disease burden and manifestations, a one-size-fits-all approach to regimen composition, dosing, and treatment duration for pediatric MDR-TB in both practice and trials may result in under-treatment or over-treatment of many children.
Pharmacokinetics of second-line anti-TB medications in children
The approach to studying individual anti-TB medications in children, has been to perform PK and safety studies, to establish doses in children that will achieve exposures similar to those in adults receiving standard doses, and safety at those doses. Extrapolation of mg/kg doses directly from adults to children is often inappropriate because of age-related changes in drug disposition and metabolism, also known as “developmental pharmacology.”; specific studies are therefore needed in children across the age spectrum (with a particular focus on very young children in whom drug handling is rapidly changing), and many important knowledge gaps remain.21 Emerging evidence on fluoroquinolone PK in children with MDR-TB has shown much lower exposures in children relative to adults with currently recommended doses.22,23,24 Age-specific PK data for ethionamide, terizidone, and para-aminosalicylic acid (PAS) are expected soon (MDRPK1 study).
Research priorities should be centered on those medications expected to be components of novel MDR-TB regimens; this includes levofloxacin, moxifloxacin, linezolid, clofazimine, and the novel medications bedaquiline and delamanid. Work on optimising pediatric doses of levofloxacin, moxifloxacin, and linezolid is ongoing (MDRPK2). Data on the PK and safety of delamanid in children aged 6–17 years have been disseminated, with work ongoing in younger children, including with a pediatric formulation. Pediatric bedaquiline studies are just starting. Clofazimine PK is poorly understood in adults, and no data for children are available, representing an important gap. Of note, PK parameters and values associated with optimal efficacy for second-line drugs are poorly-defined for adults, so PK targets for children are not well established. In general, dose-finding studies aim to identify doses that give equivalent exposures in adults and children. However, despite “low” drug exposures of key medications like the fluoroquinolones, outcomes in children with MDR-TB are good relative to adults.16 This suggests that children may need less intense treatment and adds justification for an efficacy trial of a shortened regimen in children with MDR-TB. Few child-friendly formulations of second-line anti-TB medications exist, however they are urgently needed to allow accurate and acceptable dosing to children in the field.
Design, sample size, and statistical considerations for MDR-TB trials in children
As with other aspects of TB trials, there are similarities and differences between studies of children and adults. Phase 3 studies of TB regimens are typically designed as superiority or non-inferiority trials. Though a number of design innovations have been proposed to increase information gained and/or efficiency, specifically MAMS designs25,26 and adaptive randomization,27 these designs are dependent on an easily identifiable intermediate outcome measure such as 2-month sputum culture conversion. Since this endpoint cannot be measured in many children, the usefulness of such innovations in trial design for studies in children may be limited.
A design issue that is of greater relevance in children is that of stratification by factors that are likely to influence treatment outcomes. Since age, extent or type of disease, and severity of disease are very variable in children, these factors should be controlled for by stratification. If regimens’ effectiveness is expected to vary by these factors, it may be necessary to perform separate sample size calculations for each stratum. In some situations a factorial design may be employed to achieve greater efficiency, but this depends on effects being similar across strata.
An issue that is more prominent in pediatric trials is the presence of imperfect final stage outcomes. By this we are referring to the lack of clarity about whether a patient’s TB has been cured. If the diagnosis was a clinical one (and was not confirmed microbiologically), or if a microbiologically confirmed diagnosis required invasive procedures to establish, it may not be possible to confirm that the disease has been eradicated; a long post-treatment observation period without relapse may give some certainly, but at the cost of a very prolonged study timeframe and consequent delay in determining the success of the investigational treatment. A final, more practical issue faced in TB trials is the inability to blind the study or provide for placebo control for some study agents. For example, replacing the injectable with an equally effective oral drug is highly desirable; however, a placebo injectable raises ethical issues, nor would it be likely acceptable to patients and families.
Selection of study population, design, and treatment arms for a trial of a novel pediatric MDR-TB regimen
Which children to include?
Consideration could be given to treating all children less than 18 years of age (the near universal age of majority) which includes adolescents, who are frequently neglected and for whom safety is rarely established. Alternatively, one might include all children less than 15 years of age, to align with the age brackets used by WHO for reporting TB statistics. Finally, a younger age cut off could be considered to try to capture those children whose pathophysiology (and drug handling) is most different from adults. Including all children, irrespective of extent of disease, is more inclusive and representative. However, specific issues exist around the treatment of children with more limited, paucibacillary disease, where shorter, less intensive regimens may be possible and for which there are clear differences in response to treatment compared to adults. A useful classification system has been proposed by Wiseman et al. which provides guidance on how to classify children as having severe vs. non-severe disease.28 It may be appropriate to include only children with a confirmed diagnosis (i.e. microbiologic confirmation of the presence of Mycobacterium tuberculosis shown to be resistant by genotypic or phenotypic testing) as this gives an unambiguous entry point and allows changes in microbiological status to provide microbiological endpoints. However, this excludes the majority of children with MDR-TB for whom the diagnosis is made clinically. A trial that included only microbiologically-confirmed cases (in whom disease severity or bacterial burden is often higher) would not be representative of all children with MDR-TB. Regarding drug resistance profile, it may be appropriate to only include children with MDR-TB with preserved susceptibility to the fluoroquinolones and injectables, as this is a more homogeneous population, and regimens (both control and intervention) could be standardised.
Trial Design
It may be appropriate to use the same control and intervention regimens for all children in the trial as this will allow simplicity, improved power to determine endpoints and transferability into practice. However, it would likely mean that many children will be over-treated (children with limited disease and less extensive resistance) and some may be undertreated (children with extensive disease and more extensive resistance). Alternatively, it may be possible to divide children in the trial into different categories (based on resistance profile, extent of disease or whether the diagnosis is microbiologically confirmed or not) and provide different intervention and control arms to each.
Composition of regimens
For the control arm, a number of options are available. First, a standard-duration, traditional WHO-recommended regimen could be selected, where all children in the trial receive the same drugs for the same duration. Standard treatment includes up to six months of an injectable and a total duration of 18 months of therapy. A second option is for all children to have an individualized control regimen whose component drugs and treatment duration is designed based on each patient’s disease severity, drug resistance profile, and response to treatment. Third, a number of distinct, pre-defined control regimens could be used based on resistance profile or severity. Finally, the new WHO-endorsed shortened regimen could be used. This has the advantage of being a 9–12 month regimen, which may be more desirable for patients and also for standardisation of study endpoints. However, there is limited experience using this regimen in children, and it is currently only recommended for patients who have TB caused by isolates that are known to be susceptible to fluoroquinolones and injectable agents or for whom resistance to these drug classes is unlikely.
When designing the intervention regimen it is important to construct a combination regimen that includes drugs that, together, achieve the following goals: 1) good early bactericidal activity, 2) potent sterilizing activity, 3) robustness to resistance, and 4) adequate penetration into relevant sites of disease. Regimens with limited drug-drug interactions, both with companion TB drugs and also with antiretroviral drugs, is also highly desirable. Finally, it is important to consider how easy the regimen would be to use programmatically, in terms of procurement, formulations, requirement for laboratory or safety testing, shelf life, etc. A fluoroquinolone (likely levofloxacin because it has limited effect on the QT interval) plus a novel drug (delamanid or bedaquiline), together with linezolid and clofazimine provides a potential core set of drugs in such a regimen. The fluoroquinolone provides potent bactericidal activity and reduces bacterial burden quickly, the novel drugs have good sterilizing activity, linezolid has a high barrier to resistance and protects companion drugs, while clofazimine has good sterilizing activity. The addition of other drugs, such as ethionamide, cycloserine, pyrazinamide and/or high dose isoniazid can be considered following careful assessment of the potential benefits versus safety risks. The duration of therapy in the intervention arm would need to be considered. With multiple active drugs, some with good sterilising efficacy, a shorter duration of therapy is a realistic possibility. Also, given that children frequently have paucibacillary disease, a shortened treatment of as few as six months may be more likely to be successful in children than adults.
Practical aspects of a pediatric MDR-TB treatment trial
For pediatric MDR-TB research, disease severity must be carefully collected and documented as disease severity will assuredly influence treatment outcomes. End points for such trials should include sub-analyses of patients with culture-confirmed disease looking at bacteriologic cure, even if the main study outcome is favorable versus unfavorable outcomes. Other measures of treatment response may include weight gain, clinical improvement (symptoms/physical signs), radiologic improvement, and changes in potential biomarkers. Given that the adverse effects (AEs) associated with individual drugs are fairly well-described and standard treatment commonly causes significant toxicity, it is especially important to carefully measure and report safety outcomes for new versus control regimens in all pediatric MDR-TB trials. Lastly, every effort should be made to confirm the presence of MDR-TB in enrolled patients (to avoid misdiagnosis or misclassification), by employing multiple diagnostic methods, including culture and phenotypic DST as well as molecular methods such Xpert and LPA.
Integrating children into adult TB trials
Despite substantial urging by paediatricians, clinical trialists and regulatory authorities, subjects under the age of 18 are rarely included in phase 3 clinical trials of TB. A recently completed trial of treatment of TB infection, the PREVENT TB Trial, was successful in enrolling adults and children as young as 2 years of age and provides an instructive example of both the challenges and some potential solutions to this problem.29 PREVENT TB was a randomized, open-label, non-inferiority trial of once-weekly, directly-observed rifapentine + INH for three months (3HP) compared to daily self-administered INH for nine months (9H) taken for the treatment of latent TB infection in high-risk tuberculin skin-test reactors. The target population was tuberculin skin-test (TST) positive close contacts of a culture-confirmed TB case; TST-converters; HIV-infected persons with a positive TST or close contacts to a TB case regardless of TST; and TST-positive persons with fibrosis on chest radiograph consistent with prior untreated TB. The primary aim of the study was to evaluate the effectiveness of weekly 3HP versus daily 9H in preventing progression to TB disease.
The study started enrolling adults and children aged 12–17 in 2001, as there were no PK data available to guide dosing in younger children. Doses were subsequently established for younger children in PK/safety studies, and in 2005 the protocol was amended to include children aged 2–11. Final accrual of children was achieved by 2010, and collaboration with a pediatric clinical trials network (IMPAACT) facilitated rapid enrolment of a large number of children. The study found 3HP to be as well-tolerated and as effective as 9H for preventing TB in children; 3HP had significantly higher treatment completion rates and was less hepatotoxic. Revision of the CDC LTBI guidelines to allow 3HP for children ages 2–11 years is now under consideration. Ideally, children should be included from the outset. However, if this is not possible, it may be possible to start the trial in adults but with a clear plan to gather some PK and safety data while the trial starts, and then include children in a planned way after a year or two. It would also be possible to do age de-escalation where adults are initially included, with older children then younger children included later. There is little reason to exclude >12 year olds from any adult trial.
Conclusions
The topics identified in this report identify the critical issues in pediatric MDR-TB that need to be addressed and provide a blueprint for moving forward. Investigators and funding agencies need to respond to this agenda so that important studies can be implemented, leading to improved treatment for children with MDR-TB.
Acknowledgments
This work was supported by RESIST-TB, IMPAACT, Vital Strategies, and New Ventures. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest: No competing interest declared.
Ethical Approval: Ethical approval was not required for the writing of this article.
References
- 1.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 3.Iseman MD. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329(11):784–91. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 4.Wallis RS, Maeurer M, Mwaba P, et al. Tuberculosis–advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis. 2016;16(4):e34–46. doi: 10.1016/S1473-3099(16)00070-0. [DOI] [PubMed] [Google Scholar]
- 5.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–18. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang S, Yao L, Hao X, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015;60(9):1361–7. doi: 10.1093/cid/civ027. [DOI] [PubMed] [Google Scholar]
- 8.Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45(1):161–70. doi: 10.1183/09031936.00035114. [DOI] [PubMed] [Google Scholar]
- 9.Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723–32. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 10.RESIST-TB. 2016 (Accessed September 29, 2016, at http://www.resisttb.org/?page_id=1602.)
- 11.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367(4):348–61. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 12.Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13(6):529–39. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 13.Zignol M, Sismanidis C, Falzon D, Glaziou P, Dara M, Floyd K. Multidrug-resistant tuberculosis in children: evidence from global surveillance. Eur Respir J. 2013;42(3):701–7. doi: 10.1183/09031936.00175812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383(9928):1572–9. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–1201. doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 16.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):449–56. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality among children diagnosed with tuberculosis: Systematic review and meta-analysis. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)30474-1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92(5):687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 19.Prideaux B, Via LE, Zimmerman MD, et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 2015;21(10):1223–7. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis. 2015;211(3):S83–95. doi: 10.1093/infdis/jiv183. [DOI] [PubMed] [Google Scholar]
- 21.Kearns GL, et al. Developmental Pharmacology — Drug Disposition, Action, and Therapy in Infants and Children. N Engl J Med. 2003;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Prats AJ, Draper HR, Thee S, Dooley KE, McIlleron HM, Seddon JA, et al. Pharmacokinetics and Safety of Ofloxacin in Children with Drug-Resistant Tuberculosis. Antimicrob Agents Chemother. 2015;59(10):6073–9. doi: 10.1128/AAC.01404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thee S, Garcia-Prats AJ, Draper HR, McIlleron HM, Wiesner L, Castel S, et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis. 2015;60(4):549–56. doi: 10.1093/cid/ciu868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thee S, Garcia-Prats AJ, McIlleron HM, Wiesner L, Castel S, Norman J, et al. Pharmacokinetics of Ofloxacin and Levofloxacin for Prevention and Treatment of Multidrug-Resistant Tuberculosis in Children. Antimicrob Agents Chemother. 2014;58(5):2948–51. doi: 10.1128/AAC.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies GR, Phillips PP, Jaki T. Adaptive clinical trials in tuberculosis: applications, challenges and solutions. Int J Tuberc Lung Dis. 2015;19(6):626–34. doi: 10.5588/ijtld.14.0988. [DOI] [PubMed] [Google Scholar]
- 26.Bratton DJ, Phillips PP, Parmar MK. A multi-arm multi-stage clinical trial design for binary outcomes with application to tuberculosis. BMC Med Res Methodol. 2013;13:139. doi: 10.1186/1471-2288-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cellamare M, Ventz S, Baudin E, Mitnick CD, Trippa L. Bayesian response-adaptive trial in tuberculosis: The endTB trial. Clin Trials. 2016 doi: 10.1177/1740774516665090. pii: 1740774516665090. [DOI] [PubMed] [Google Scholar]
- 28.Wiseman CA, Mandalakas AM, Kirchner HL, Gie RP, Schaaf HS, Walters E, Hesseling AC. Novel application of NIH case definitions in a paediatric tuberculosis contact investigation study. Int J Tuberc Lung Dis. 2015;19(4):446–53. doi: 10.5588/ijtld.14.0585. [DOI] [PubMed] [Google Scholar]
- 29.Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169(3):247–55. doi: 10.1001/jamapediatrics.2014.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]


