Figure 1.
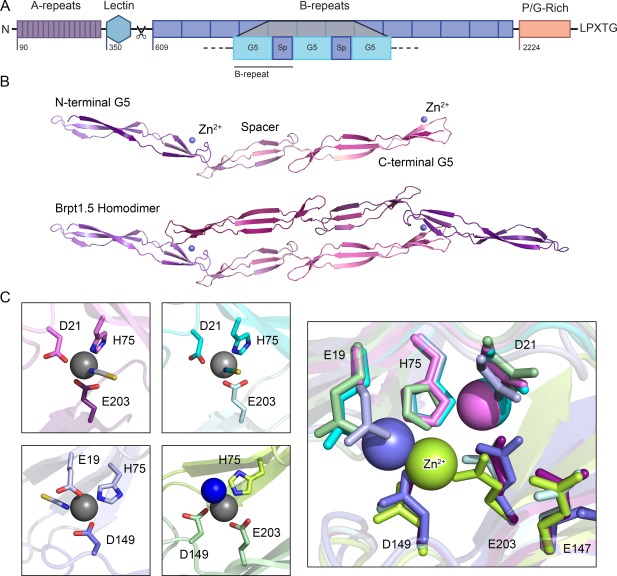
An overview of the structural characteristics of Aap. (A) The distinct regions of Aap include 16 short A‐repeats, a globular lectin domain, between 5–17 tandem B‐repeats (each of which contains a metal‐binding G5 domain followed by a spacer domain, denoted as “Sp”), a proline/glycine‐rich stalk region and a LPXTG cell wall anchoring motif. The A‐repeats and lectin domains are proteolytically processed to expose the B‐repeat superdomain, which can then assemble with B‐repeat regions on other staphylococcal cells to mediate intercellular adhesion in the biofilm. (B) Previously solved crystal structures of the Brpt1.5 construct showing the monomer alone and the Zn2+‐induced dimer.7, 8 Each G5 domain consists of two free‐standing, 3‐stranded β‐sheets separated by a triple‐helix‐like twist; the spacer domain adopts a similar but shorter version of the same fold. The metal‐binding sites are found on the face of the second β‐sheet in each G5 domain (identified by a blue metal ion, labeled Zn2+). (C) Dimerization occurs upon coordination of the Zn2+ ion in trans, creating a tetrahedral binding site with two ligands from one G5 domain and a third ligand from the opposite protomer. The fourth site is occupied by a solvent molecule. Comparing four crystal structures of Brpt1.5 bound to Zn2+, we observed three distinct coordination schemes, each of which was validated in solution by mutagenesis and analytical ultracentrifugation.7 This pleomorphism in metal ion coordination is likely the underlying basis for the ability of the B‐repeat region to self‐assemble with either Zn2+ or Cu2+.
