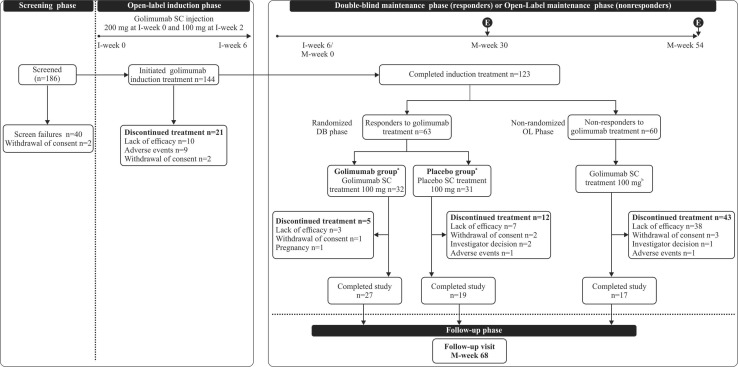
Fig. 1.
Study design and patient disposition. E Primary efficacy evaluation, DB double-blind, I-week induction week, M-week maintenance week, OL open-label, SC subcutaneous; a every 4 weeks through M-week 52; b patients who responded to golimumab 100 mg at M-week 8 continued to receive golimumab 100 mg every 4 weeks through M-Week 52 at the same dose