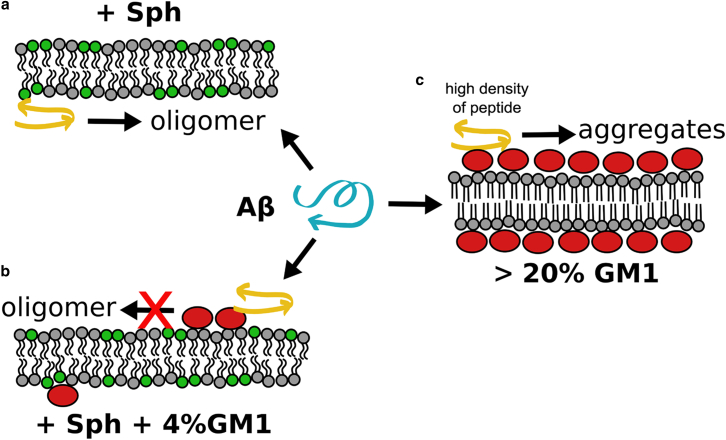
Figure 3.
Formation of toxic Aβ oligomers can be differently influenced by different local GM1 organization. Proposed model: (a) and (b) depict the inhibitory effect of low concentrations of GM1, organized in low-density and dynamic nanoclusters, on the membrane-mediated oligomerization of Aβ (membrane-bound peptide is symbolized by the yellow arrows). (a) Membrane-mediated oligomerization of low nanomolar solution concentrations of Aβ (Aβ/total lipid ratios of 1:4000, at maximum) is triggered by the presence of sphingomyelin (green lipids; print version: light gray) (25). (b) Binding of Aβ to the headgroup of GM1 (red ellipses) sequesters the peptide and prevents it from oligomerizing (25). (c) High concentrations of GM1, organized in high-density platforms of slow dynamics, create a surface that facilitates the adsorption and local concentration of Aβ. At high Aβ densities, oligomerization of the peptide is promoted (42, 43), which is a generic effect also observed for other surfaces (54, 55). Thus, in the low GM1 concentration regime (also low local Aβ concentration) the ganglioside acts as an inhibitor of membrane-mediated oligomerization. In the high GM1 concentration regime—where the membrane is covered by GM1 (21)—the ganglioside can become a catalyst of oligomerization, depending on the amount of Aβ on the surface of the GM1 platform. To see this figure in color, go online.