Fig. 1.
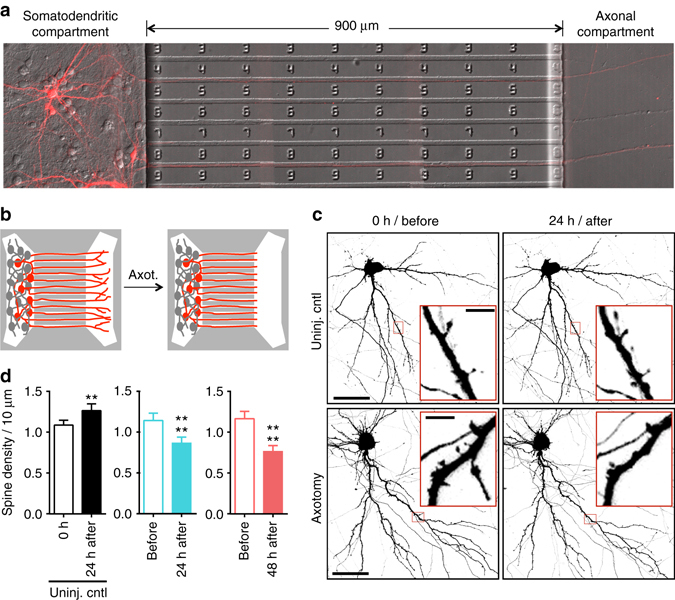
Dendritic spine density of pyramidal neurons within microfluidic chambers before and after axotomy. a 14 DIV rat hippocampal neurons cultured within a microfluidic chamber. Pyramidal neurons were retrogradely labeled using a G-deleted mCherry rabies virus added exclusively to the axonal compartment. b Cartoon illustration of in vitro axotomy (Axot.) within microfluidic chambers to selectively axotomize a subset of labeled neurons (red) that extend axons through the microgroove region. Axons of neighboring uncut neurons (gray) remain within the somatodendritic compartment. c Representative images of mCherry-labeled neurons and dendritic segments (inverted fluorescence) from repeated live imaging of uninjured control chambers (Uninj. cntl) and axotomized chambers. Axotomized chambers were imaged before axotomy on 13 DIV (before) and uninjured control chambers were also imaged on 13 DIV (0 h); both conditions were then imaged at 14 DIV (24 h after). Image and inset scale bars, 50 and 5 µm, respectively. d Quantification of spine density illustrated in c. Uninj. cntl: n = 20 dendrites; 5 neurons; 3 chambers over 3 experiments; #spines/TDL: 315/2698 µm (0 h), 388/2737 µm (24 h after). Axot.: n = 26 dendrites; 5 neurons; #spines/TDL: 405/3569 µm (before), 274/2998 µm (24 h after), and 229/2821 µm (48 h after). 3 chambers over 3 experiments. TDL: total dendritic length. Paired two-tailed t-test. **p < 0.01, ****p < 0.0001. Error bars, s.e.m
