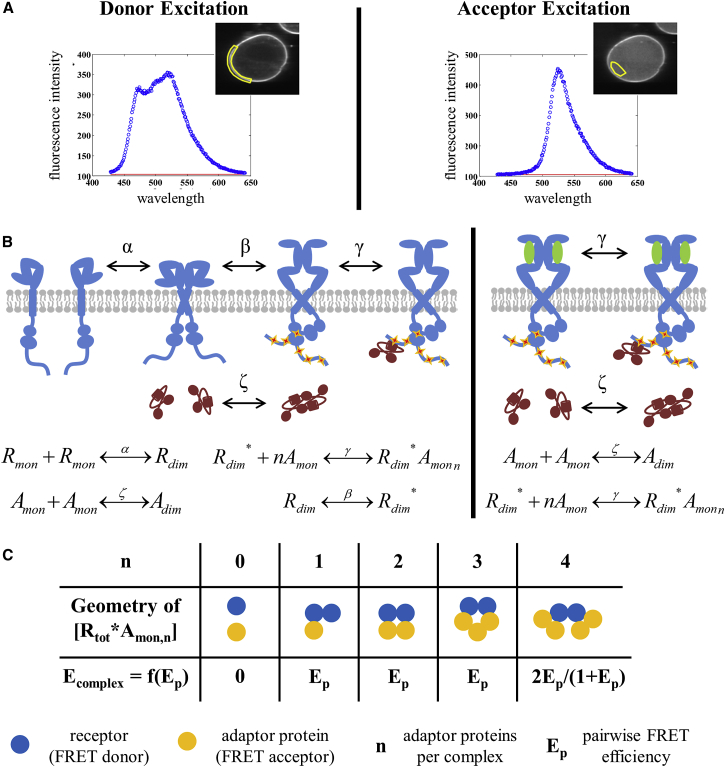
Figure 1.
An overview of the methodology used to quantify the interactions between membrane proteins and cytoplasmic proteins. (A) Representative images (insets) of a CHO cell under reversible osmotic stress and fluorescence emission spectra collected using the FSI technique. FSI yields approximation-free measurement of donor (EGFR-mTurquoise) concentration, acceptor (Grb2-YFP) concentration, and FRET efficiency in selected regions of a cell under reversible osmotic stress (see (37) for details). (B) Cartoon representation of the thermodynamic equilibrium reactions in the presence of saturating concentrations of the EGF ligand (right) and in the absence of ligand (left). The receptors are shown in blue, the ligand in green, the phosphorylation sites in orange, and the adaptor proteins in red. The adaptor protein Grb2 exists in a monomer-dimer equilibrium in the cytoplasm, where the dimerization constant ζ is known (23, 51). EGFR kinase domains are believed to adopt either an active asymmetric dimer configuration that is phosphorylated and capable of interacting with Grb2, or an inactive symmetric configuration (64, 65, 66). In the presence of saturating amounts of ligand, we assume that all receptors exist as active dimers that are either free or bound to Grb2, and we determine the association constant, γ. In the absence of ligand, we assume that the receptors are found as monomers, inactive dimers, active dimers, and RTK dimer-adaptor monomer complexes. We determine the conformational parameter, β, describing the transition from the inactive dimer to the active dimer configuration. (C) Possible oligomer sizes and geometries for the assembly of an RTK dimer with monomeric adaptor proteins. The FRET efficiency, Ecomplex, in each complex of fluorophores is calculated as a function of the pairwise FRET efficiency, Ep, as described previously (74). To see this figure in color, go online.