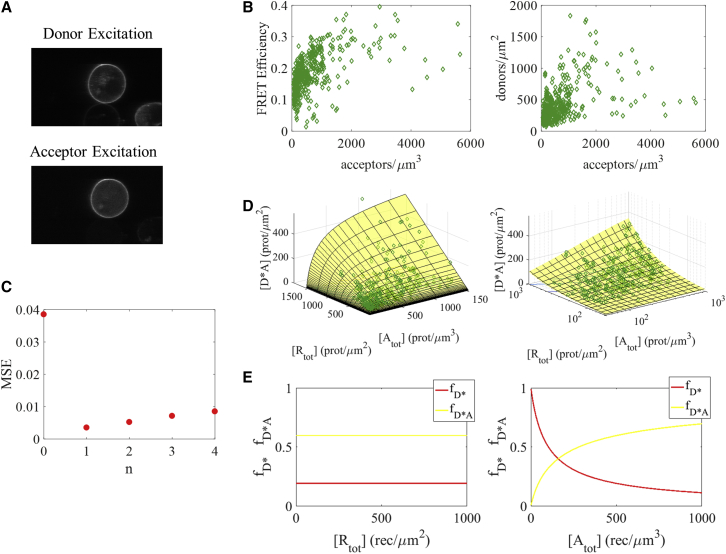
Figure 2.
FRET data for the interaction between EGFR and Grb2, under saturating ligand conditions. (A) A representative image of a CHO cell under reversible osmotic stress, in the presence of EGF. Fluorescence in the donor excitation image indicates the location of EGFR; fluorescence in the acceptor excitation image denotes Grb2. (B) In the left-hand plot, FRET efficiency in the plasma membrane is shown as a function of Grb2 (acceptor) concentration in the cytoplasm. The right-hand plot shows EGFR (donor) concentration in the membrane versus Grb2 (acceptor) concentration in the cytoplasm. (C) The MSE for fits of Eq. 20 to the experimental data when the number of monomeric adaptor proteins, n, is varied between 0 and 4. The model with one Grb2 monomer per EGFR dimer gives the best fit. (D) The theoretical and the experimental concentrations of the RTK dimer-adaptor monomer complex, [Rtot∗Amon,n], are calculated using Eqs. 18 and 19, respectively. The best-fit model (yellow surface) and experimental data (green diamonds) are plotted as a function of both total receptor concentration, [Rtot], and adaptor protein concentration, [Atot], on linear (left) and semi-log (right) scales. (E) The fraction of receptors found in active dimers with no adaptors bound (red line) and in RTK dimer-adaptor monomer complexes (yellow line) for the best-fit model as a function of total receptor concentration (left) and adaptor protein concentration (right). To see this figure in color, go online.