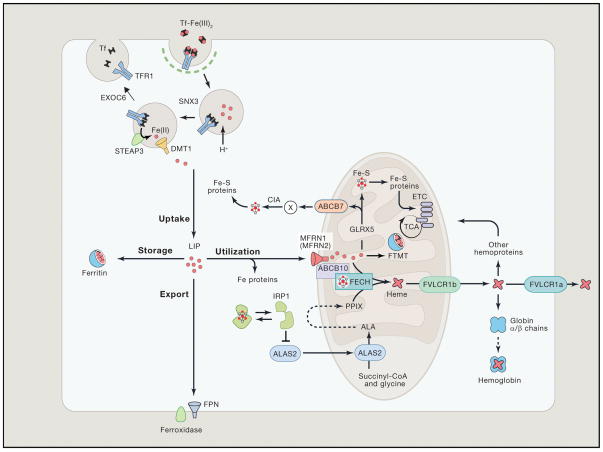
Figure 1. Iron Metabolism in Erythroid Cells.
Most of the daily amount of iron needed for erythropoiesis is recycled by specialized macrophages in the spleen and liver. Iron acquisition in erythroid cells is dependent on endocytosis of diferric transferrin (Tf-Fe2) via the transferrin receptor (TFR1). In acidified endosomes, iron is freed from Tf and exported into the cytoplasm by DMT1 after reduction of the metal by STEAP3 (six-transmembrane epithelial antigen of prostate 3). The recycling of Tf and TFR1 is crucial for optimal iron uptake and requires SNX3 (sortin nexin 3) and EXOC6 (exocyst complex component 6 also known as Sec15L1). The metabolically active labile iron pool is used directly for incorporation into iron proteins or transported into mitochondria via mitoferrin 1 (MFRN1, MFRN2 in non-erythroid cells), which forms a complex with ABCB10 and FECH. In mitochondria, iron is inserted into protoporphyrin IX (PPIX) by ferrochelatase (FECH) to produce heme. Heme is transported out of the mitochondria via the 1b isoform of FLVCR (feline leukemia virus subgroup C cellular receptor) for incorporation into hemoproteins, mostly hemoglobin; the FLVCR1a isoform exports heme from the cytosol. Mitochondrial iron is also used for Fe-S cluster synthesis, which among other factors involves GLRX5 (Glutaredoxin-related protein 5). Fe-S clusters are incorporated into client proteins throughout the cell. Maturation of cytosolic Fe-S proteins by the CIA (cytosolic Fe-S cluster assembly) system depends on an unknown compound (designated X) generated during mitochondrial Fe-S cluster biosynthesis. Its export into the cytosol requires the ATP-binding cassette protein ABCB7. Fe-S proteins play key roles in the regulation of iron metabolism. For instance, under conditions of iron or GLRX5 deficiency, iron regulatory protein 1 (IRP1) is devoid of a Fe-S cluster and inhibits translation of ALAS2, the first and rate-limiting enzyme of heme biogenesis, thereby preventing the accumulation of toxic heme intermediates. In the mitochondria, excess iron can be stored in the organelle-specific form of mitoferritin (FTMT). In the cytosol, excess iron is sequestered within heteropolymers of ferritin H and L chains. Cellular iron efflux is mediated by ferroportin (FPN) and requires iron oxidation on the extracellular side. Reprinted with permission from Muckenthaler et al.20 and Cell (Elsevier, Inc).