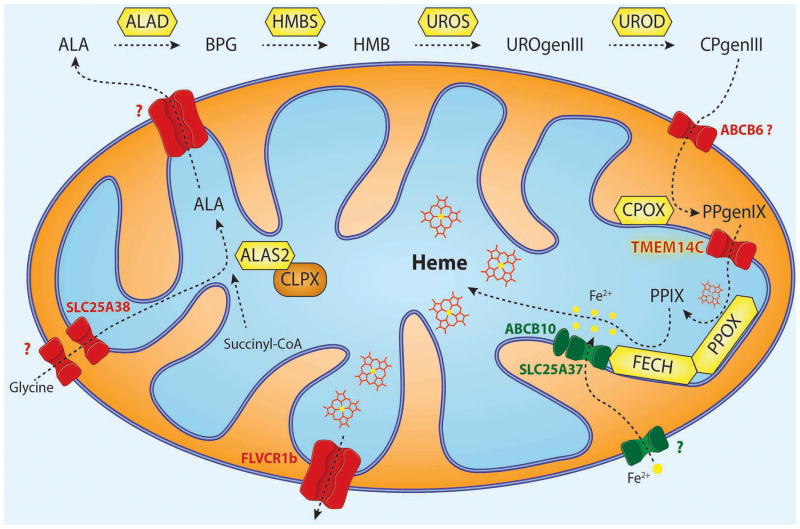
Figure 2. Intracellular trafficking of heme intermediates in erythroid cells.
Glycine is imported via SLC25A38 and condenses with succinyl-CoA to form δ-aminolevulinic acid (ALA) in a reaction catalyzed by ALA synthase (ALAS2 in red cells).75 ALAS is activated by mitochondrial chaperone ClpX through promoting the incorporation of pyridoxal phosphate, an essential cofactor for ALAS function.72 After several catalytic conversions of heme precursors, coproporphyrinogen III (CPgenIII) is transported into the mitochondrial intermembrane space. It is then converted to protoporphyrinogen IX (PPgenIX) that is transported into the matrix by a mechanism requiring TMEM14C.77 Ferrochelatase (FECH) metallates protoporphyrin IX (PPIX) with iron to form heme. Studies have shown that iron enters the mitochondrial matrix via mitoferrin-1 (SLC25A37) in the inner mitochondrial matrix.37 SLC25A37 is stabilized by ABCB10 and exists in a large oligomeric complex with FECH.46,47 Heme is thought to be exported by FLVCR1b from the mitochondria into the cytosol, where it is incorporated into hemoproteins.57,58 Figure illustration courtesy of Johannes G. Wittig (Technische-Universität-Dresden, Germany). Reprinted with permission from Yien et al.65 and Oncotarget (Impact Journals, LLC).