Figure 3. Loss of Fe–S cluster production interferes with IRP1-mediated intracellular iron homeostasis.
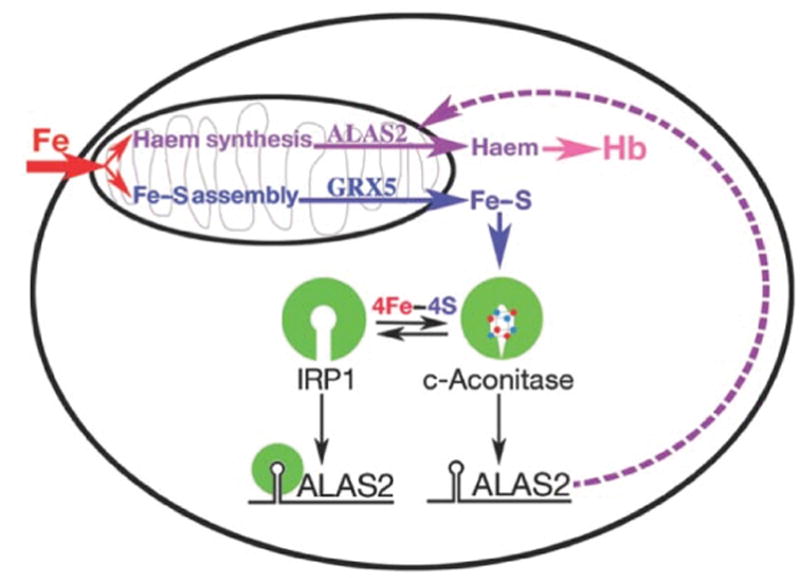
Model for the role of Fe–S cluster production in erythroid heme synthesis. Iron imported into mitochondria is used in the independent pathways of heme and Fe–S cluster biogenesis. Normal production of Fe–S clusters, which requires Glrx5, switches the bifunctional protein IRP1 to a cytoplasmic aconitase (c-aconitase); this permits ALAS2 protein synthesis needed for heme production. In the absence of Fe–S clusters, IRP1 has IRE-binding activity, and binds the 5′-IRE on ALAS2 mRNAs, blocking translation and thereby preventing heme production. Reprinted with permission from Wingert et al.82 and Nature (Macmillan Publishers, Ltd).
