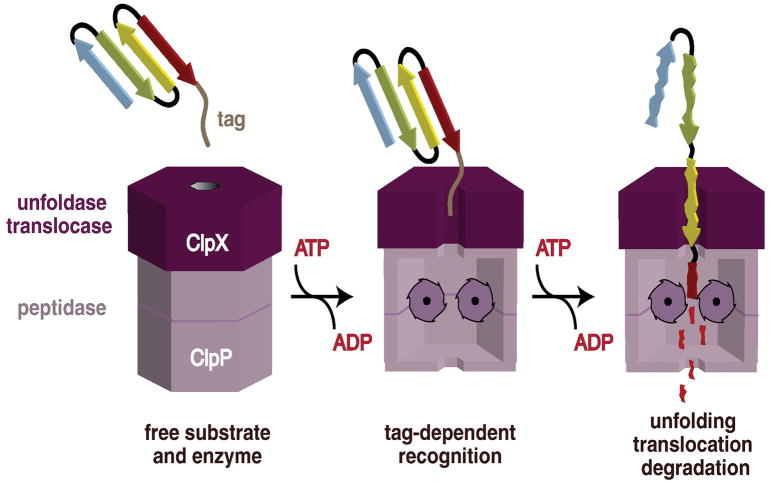
Figure 5. Model of Substrate Recognition and Degradation by a AAA+ Protease, ClpX.
Cartoon model of substrate recognition and degradation by the ClpXP protease. ClpXP is composed of both ClpX and ClpP, which function as a hexameric unfoldase and tetradecameric peptidase, respectively. In an initial recognition step, a peptide tag in a protein substrate binds in the axial pore of the ClpX hexamer. In subsequent ATP-dependent steps, ClpX unfolds the substrate and translocates the unfolded polypeptide into the degradation chamber of ClpP for proteolysis, where it is cleaved into small peptide fragments. Reprinted with permission from Baker et al 99 and Biochimica et Biophysica Acta (Elsevier, Inc).