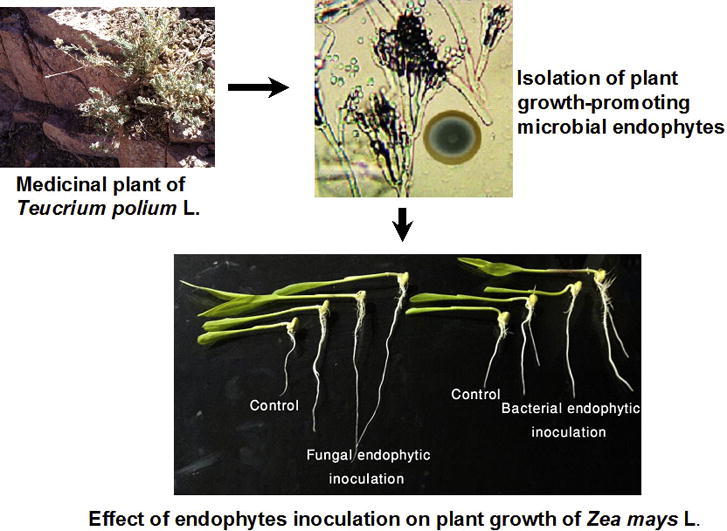
Graphical abstract
Keywords: Antimicrobial activity, Endophyte, IAA, Phosphate solubilization, Plant growth promoting bacteria, Teucrium polium L., Zea mays L.
Abstract
Bacterial and fungal endophytes are widespread inhabitants inside plant tissues and have been shown to assist plant growth and health. However, little is known about plant growth-promoting endophytes (PGPE) of medicinal plants. Therefore, the aims of this study were to identify bacterial and fungal endophytes of Teucrium polium and to characterize plant growth-promoting (PGP) properties of these endophytes. Seven bacterial endophytes were isolated and identified as Bacillus cereus and Bacillus subtilis, where five endophytic fungi were obtained and assigned to Penicillium chrysogenum and Penicillium crustosum. The isolated endophytes differentially produced indole acetic acid (IAA) and ammonia, and in addition to their enzymatic and antimicrobial activities, they exhibited variable capacity for phosphate solubilization. In order to investigate the effect of endophytes on plant growth, four representative endophytes and their consortiums were selected concerning to their potential ability to promote plant growth. The results indicated that microbial endophytes isolated from medicinal plants possessing a vital role to improve plant growth and could be used as inoculants to establish a sustainable crop production system.
Introduction
The long-term approaches of organic and inorganic fertilizers beside to pesticides are urgently required for the enhancement of crop production [1]. Notably, these applications negatively influence on soil quality and contribute to environmental pollution [2]. Concerning to minimize the detrimental effects of the conventional techniques of agriculture, innovative methods based on microbial inoculation are recently gaining more interest. Plants and microorganisms form a symbiotic association with benefits for both partners. More importantly, plant-microbe symbiosis influence on plant growth and health which effectively ameliorates agricultural traits and improve soil quality and nutrient cycling [3], [4], [5].
Plant growth-promoting endophytes (PGPE) inhabit plant tissues and the close linkage of endophytes inside plant tissues facilitates nutrients exchange and enzymes activity [6], [7]. The distribution of growth-promoting hormones produced by endophytic microorganisms towards plant tissues positively promotes plant growth [8]. Endophytes possess vital ability to mobilize insoluble phosphate and provide nitrogen to their host plants [9], [10]. Microbial endophytes colonize plant tissues without symptomatic behaviour and consequently they compete with other microbial pathogens on the same ecological niches. Therefore, the established plant-endophyte association improves plant health via different mechanisms displayed by endophytes and potentially contributes the protection of plant host against microbial pathogenesis [11]. PGPE produce various bioactive compounds with several biological activities which can be directly or indirectly described as plant growth-promoting (PGP) agents. Approximately most of the plants harbour endophytes interior their tissues; however, available information on PGPE and their biological activities is not equivalent to the high distribution of endophytes. A superior comprehension of the native endophytes of plants may help clarify their capacities and potential in enhancing plant growth and establishing a sustainable system for crop production.
Teucrium polium L. is a wild plant belonging to family Lamiaceae and naturally found in Saint Katherine Protectorate which is a part from the Sinai Peninsula in Egypt. Saint Katherine Protectorate is characterized by extremely arid climate and it is located at average of 1500–2000 m above mean sea level [12]. It has been reported that T. polium plant used in traditional medicine as antimicrobial and antiseptic; more recently, several bioactive compounds such as terpenoids and flavonoids have been isolated from T. polium and their pharmaceutical uses as antimicrobial, antioxidant, and anticancer have been investigated [13]. Although the ecological and biological importance of T. polium as a medicinal plant inhabitant in unique environmental area, microbial endophytes of this plant have been yet investigated. A previous study focused on the isolation of fungal endophytes from T. polium which occurs in Saint Katherine Protectorate [12]; screening antimicrobial activities of these fungal endophytes have only been examined. Till now, no published study has been inspected plant growth-promoting activities of microbial endophytes correlated to this medicinal plant in natural arid habitats. Discovery the presence of endophytic bacteria and fungi in T. polium could be biotechnologically applicable; therefore, hypothesis of the current study is that microbial endophytes of this medicinal plant are promising bio-inoculants for plant growth promotion trait. The outline of this study concentrates on the isolation, molecular identification, and characterization of putative bacterial and fungal endophytes related to medicinal plant of T. polium which naturally occur in arid conditions of Saint Katherine Protectorate. More specificity, verification for plant growth-promoting properties of these microbial isolates such as enzymatic production, antimicrobial activity, IAA and ammonia production, and P solubilization were evaluated to test their influence on the biomass production of maize plant as an important economical crop.
Material and methods
Plant sampling and microbial endophytes isolation
Medicinal plant of Teucrium polium L. (family Lamiaceae) was collected from Wadi al-Zwatin (lat 28.539290° to 28.53919° N; long 33.930784° to 33.92044° E), Saint Katherine Protectorate, Sinai Peninsula, Egypt. The plant leaves were washed by running tap water and subsequently by sterile distilled water, then surface sterilized by ethanol 70% for 1 min, sodium hypochlorite 2.5% for 5 min, ethanol 70% for 30 s, and finally washed in sterile distilled water for 3 times. The last washing water was plated onto nutrient agar, Czapek Dox (CD) agar, and potato dextrose agar (PDA) media. Additionally, any microbial DNA in the last washing water was detected by the amplification of bacterial rRNA genes or fungal ITS fragments. The success of surface sterilization method was confirmed by the absence of microbial DNA amplification and also when no microbial growth was detected on the cultural media. Bacterial endophytes were isolated from the sterilized plant leaves [14] on Luria-Bertani (LB) and nutrient agar media, where fungal endophytes were isolated on PDA and CD media [6]. The bacterial and fungal growth from the internal tissues or crushed segments were checked for purity, transferred to fresh cultural slants and stored at 4 °C for further study.
Molecular identification of endophytes
Molecular identification was carried out based on bacterial 16S rRNA gene and fungal internal transcribed spacer (ITS) rDNA rejoins amplification and sequence analyses. Genomic DNA was extracted according to the method of Miller et al. [15]. Bacterial 16S rRNA genes were amplified using the genomic DNA as template and bacterial universal primers of 27 f (5-GAGTTTGATCACTGGCTCAG-3) and 1492 r (5-TACGGCTACCTTGTTACGACTT-3) [16]. Fungal ITS rDNA rejoins were amplified by the primers of ITS1 f (5-CTTGGTCATTTAGAGGAAGTAA-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) [17]. The PCR mixture contained: 1 × PCR buffer, 0.5 mM MgCl2, 2.5 U Taq DNA polymerase (QIAGEN, Germantown, MD 20874, USA), 0.25 mM dNTP, 0.5 μM of each primer, and 1 μl of extracted genomic DNA. The PCR was performed in a DNA Engine Thermal Cycler (PTC-200, BIO-RAD, USA) with 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, followed by a final extension performed at 72 °C for 10 min. The PCR products were checked for the expected size on 1% agarose gel and were sequenced at the Genome Quebec Innovation Center Facility (Montreal, Canada) with the two primers. The retrieved bacterial sequences at this study have been deposited in GenBank under accession numbers from KY905357 to KY905363, where the accession numbers of fungal ITS sequences are KY906184-KY906188. The sequences were compared against the GenBank database using the NCBI BLAST program. Multiple sequence alignment was done using ClustalX 1.8 software package (http://wwwigbmc.u-strasbg.fr/BioInfo/clustalx) and a phylogenetic analysis was constructed by the neighbor-joining method using MEGA (Version 6.1) software, with confidence tested by bootstrap analysis (1000 repeats).
Screening of plant growth-promoting properties of endophytes
Microbial endophytic isolates were screened for phosphate solubilization by procedure of Jasim et al. [18] using Pikovskaya medium and bromophenol blue as indicator. The ability of endophytic isolates to produce ammonia was assessed using Nesseler’s reagent in peptone liquid media [19]. Where, the intensity of colour change indicates the endophytes capacity for ammonia production.
Produced extracellular enzymes by bacterial endophytes were assessed with conducting the tested substrates into a mineral salt (MS) media and inoculating the media with bacterial isolates. The production of extracellular enzymes by fungal endophytes were detected by growing on Yeast-Malt (YM) agar media (YM: 10 g/L glucose, 5 g/L peptone, 3 g/L yeast extract, 3 g/L malt extract, 1.5% agar, pH 6.7) and placing 5 mm fungal plugs on the YM agar media supplemented with dissolved and specific indicative substrates. Amylase, cellulose, protealase, pectinase, and xylanase activities of endophytes were evaluated by growing the endophytic isolates on media supplemented with 1% of soluble starch, cellulose or carboxy-methylcellulose (CMC), gelatine, Pectin, and xylan, respectively. The appearance of clear zone was measured after adding specific reagents (iodine, acidic mercuric chloride, hexadecyl trimethyl ammonium bromide, and absolute ethyl alcohol to detect the amylolytic and cellulolytic, proteolytic, pectinolytic, xylanolytic activities, respectively) and used as indicator for extracellular enzymatic activities [20].
To test the antimicrobial activity of endophytes, the bacterial isolates were cultured in nutrient broth medium for 6 days at 35 ± 2 °C on a shaker at 180 rpm. Where, fungal isolates were cultured in Malt extract liquid medium for 10 days at 28 °C on a shaker at 180 rpm. Crude fermentation broth were blended thoroughly and centrifuged at 4000 rpm for 5 min. Liquid supernatant was extracted with an equal volume of ethyl acetate thrice. The organic solvent extract was then evaporated and the crude extracts were dissolved in dimethyl sulfoxide (DMSO) and used for antimicrobial screening assay by well diffusion method [21]. The coded test organisms used for antimicrobial assay were; Gram-positive bacteria: Staphylococcus aureus, ATCC 6538 and Bacillus subtilis, ATCC 6633; Gram-negative bacteria: Escherichia coli, ATCC 8739; Pseudomonas aeruginosa, ATCC 9027 and Salmonella typhimurium ATCC 14028; and yeast strain of Candida albicans, ATCC 10231. Antimicrobial activity of the endophytic crude extracts was determined by the method previously described [20].
The ability of endophytes to produce IAA was determined with Salkowski’s reagent and different concentrations of tryptophan (1, 2 and 5 mg mL−1) or without tryptophan according to the colorimetric method previously described [22]. Moreover, the highest producers for IAA were selected and subjected to another assay for detection the amount of IAA at 2 days interval within 14 days in the presence of optimum tryptophan concentration 5 mg mL−1.
Effect of the endophytic inoculation on maize growth
Two bacterial isolates of Tp.1B and Tp.6B, a bacterial consortium formed a mix of Tp.1B-Tp.6B, two fungal isolates Tp.2F, and Tp.5F, and a fungal inoculum formed a mix of Tp.2F-Tp.5F were selected for their better PGP activities in order to test the effect of endophytic inoculation on root length and the biomass productionh of maize plant. The seeds were surface disinfected by soaking in 2.5% sodium hypochlorite for 5 min, 70% ethanol for 1 min, and then washed by sterile distilled water for 5 times. Bacterial pure cultures of Tp.1B and Tp.6B were grown in nutrient broth at 35 ± 2 °C on a shaker at 180 rpm. The bacterial cultures were diluted in sterilized distilled water to reach final concentration of 106–8 CFU mL−1 [14]. Fungal isolates of Tp.2F and Tp.5F were inoculated in CD broth at 28 °C on a shaker at 180 rpm for 7 days [20]. Pregerminated and surface-disinfected seeds were incubated with bacterial or fungal suspensions at room temperature for 6 h, the broth without microbial inoculation was used to treat control seeds. Control and treated seeds were placed in sterilized Petri dishes with wet sterilized filter papers and incubated at room temperature for 5 days in dark to measure the root length. The experimental treatments were conducted in 5 replications, in which 10 seeds per each replicated unit.
A pot experiment greenhouse was carried out to evaluate the effect of endophytic inoculation on maize growth. A completely randomized design was set up including the inoculation with endophytes of Tp.1B, Tp.6B, Tp.2F, and Tp.5F plus microbial consortiums of Mix.1-6B (bacterial isolates of Tp.1B and Tp.6B) and Mix.2-5F (fungal isolates Tp.2F, and Tp.5F). The disinfected seeds were germinated on wet sterilized filter papers and incubated at room temperature for 5 days in dark. The equal sized seedling were chosen and immersed in bacterial or fungal suspensions at room temperature for 2 h [14], where control seedling were treated by free-bacterial or free-fungal suspensions. Control and treated seedling were transplanted in 1 L plastic pots filled with 900 g of sterilized soil-sand mixture, 3 seedlings per pot. A loamy soil (sand 76.8%, silt 10.9%, and clay 12.2%) with chemical characters of: pH = 6, CEC (mEq per 100 g) = 15, organic matter = 5.2%, 24 mg kg−1 P, 15.1 mg kg−1 K, 186.4 mg kg−1 Na, 27.3 mg kg−1 Ca, and 134.4 mg kg−1 Cl was used. This soil was air-dried, sieved with a 2 mm sieve, and autoclaved twice for one hour at 121 °C. For further successful inoculation, one-week-old seedlings were treated with 10 mL of microbial suspensions [23]. Control seedling were loaded by free-bacterial or free-fungal suspensions. Plants were grown in a greenhouse with temperature 25–30 °C and irrigated by tap water without adding any fertilization. After 30 days plants were harvested, shoot and root systems were separated, and roots were washed carefully with tap water to remove soil particles attached with roots. The shoots and roots fresh weight were measured, and then oven dried for 72 h at 60 °C and weighted to determine the dry weights.
Statistical analysis
Data were statistically analyzed by SPSS v17, one-way analysis of variance (ANOVA) test was used for multiple sample comparison, when normality and homogeneity of variance were satisfied, followed by multiple comparison LSD test at P < 0.05.
Results
Molecular identification of endophytes
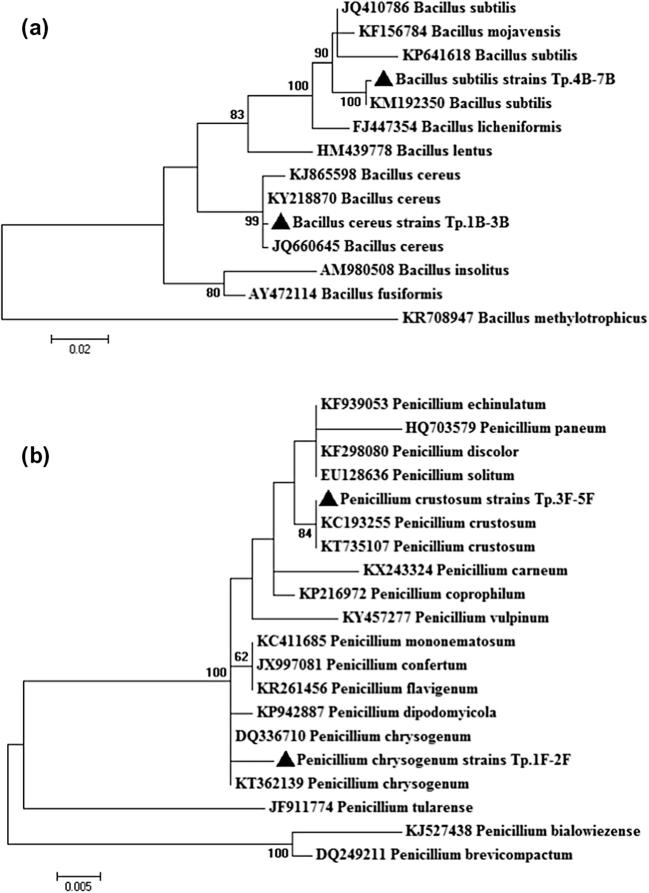
Seven bacterial endophytes were isolated and purified from the shoot system of T. polium plant. Phylogenetic analysis based on 16S rRNA gene sequencing and alignment revealed that these endophytes were classified into two different species of Bacillus (Fig. 1a). The molecular identification of 16S rRNA gene fragments of these isolates showed that, 3 isolates of Tp.1B-3B were identified as Bacillus cereus strain BVC62 (JQ660645) with 99% identity and 4 isolates of Tp.4B-7B had 16S ribosomal amplicons similar to Bacillus subtilis strain UPMB10 (KP641618) with 99% homology (Table 1). Moreover, five endophytic fungi were obtained and genotypically clustered into two different species of Penicillium (Fig. 2b). The BLAST results showed that the ITS sequences of 2 isolates Tp.1F-2F were more relatives to Penicillium chrysogenum isolate MS15 (KT362139) with 99% genetic similarity, where 3 isolates of Tp.3F-5F were affiliated to Penicillium crustosum strain 2T01Y01 (KC193255) with 98% homology (Table 1).
Fig. 1.
Phylogenetic analysis of: (a) 16S rRNA sequences of the bacterial isolates, and (b) ITS regions of fungal isolates, with the sequences from NCBI. Tp.1B-3B and Tp.3B-7B refer to 16S rRNA sequences retrieved from bacterial endophytes, where Tp.1F-2F and Tp3F-5F are the sequences obtained from fungal endophytes. The analysis was conducted with MEGA 6 using neighbor-joining method with bootstrap value (1000 replicates).
Table 1.
The molecular identification of endophytic microbial isolates from T. polium.
| Microbial isolate code | Nearest holomogue sequences (accession number) | Sequence identity % | Accession numbers | |
|---|---|---|---|---|
| Bacterial endophytes | Tp.1B | Bacillus cereus (JQ660645) | 99 | KY905357 |
| Tp.2B | Bacillus cereus (JQ660645) | 98 | KY905358 | |
| Tp.3B | Bacillus cereus (JQ660645) | 98 | KY905359 | |
| Tp.4B | Bacillus subtilis (KP641618) | 99 | KY905360 | |
| Tp.5B | Bacillus subtilis (KP641618) | 99 | KY905361 | |
| Tp.6B | Bacillus subtilis (KP641618) | 98 | KY905362 | |
| Tp.7B | Bacillus subtilis (KP641618) | 98 | KY905363 | |
| Fungal endophytes | Tp.1F | Penicillium chrysogenum (KT362139) | 99 | KY906184 |
| Tp.2F | Penicillium chrysogenum (KT362139) | 98 | KY906185 | |
| Tp.3F | Penicillium crustosum (KC193255) | 98 | KY906186 | |
| Tp.4F | Penicillium crustosum (KC193255) | 98 | KY906187 | |
| Tp.5F | Penicillium crustosum (KC193255) | 99 | KY906188 | |
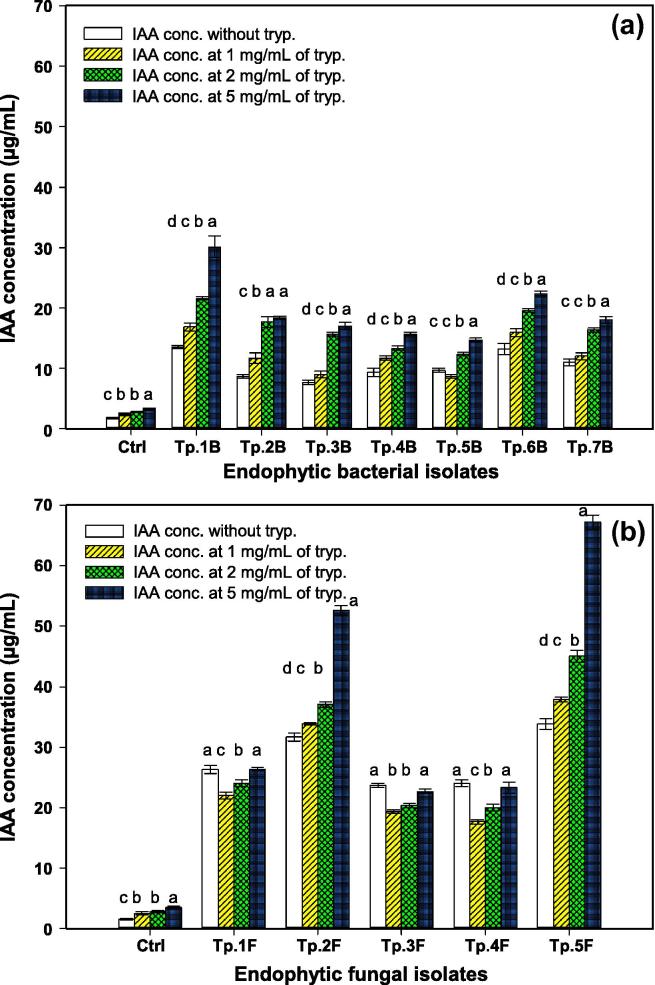
Fig. 2.
Quantitative production of IAA by: (a) endophytic bacterial isolates, and (b) endophytic fungal isolates; with and without tryptophan. Bars with the same letter for each endophytic isolates did not differ significantly at significant level of (P ≤ 0.05); Error bars indicate means ± SE by LSD test (n = 6).
Screening plant growth promoting activities of endophytes
The PGP activities of bacterial and fungal endophytes that could directly or indirectly promote plant growth and health were evaluated. The results revealed that all isolated fungal endophytes differentially produced ammonia, while 3 bacterial endophytes of Tp.2B, 4B and 5B were not be able to produce ammonia. ANOVA analysis showed that endophytic fungi displayed higher ability to solubilize inorganic phosphate than those recorded for endophytic bacteria (Table 2). Fungal isolate of Tp.2F and Tp.5F showed the highest (P = 0.01) ability for phosphate solubilization compared to other endophytes.
Table 2.
Ammonia production and phosphate solubilization of endophytic microbial isolates.
| Microbial isolates | Ammonia production | P solubilization Diameter of clear zone (mm) |
|---|---|---|
| Tp.1B | ++ | 6.3 ± 0.4d |
| Tp.2B | − | 0f |
| Tp.3B | + | 0f |
| Tp.4B | − | 3.3 ± 0.3e |
| Tp.5B | − | 0f |
| Tp.6B | ++ | 7.7 ± 0.3c |
| Tp.7B | + | 3.7 ± 0.3e |
| Tp.1F | + | 6.0 ± 0.5d |
| Tp.2F | ++ | 10.7 ± 0.3a |
| Tp.3F | + | 0f |
| Tp.4F | + | 7.3 ± 0.2c |
| Tp.5F | ++ | 9.3 ± 0.3b |
−, +, and ++ denote no, low, and high ammonia production, respectively. Values within the same column with different letters are significantly different (P ≤ 0.05) by LSD test, values are means ± SE (n = 6).
Antimicrobial activities of endophytes showed that bacterial endophyte of Tp.6B inhibited the growth of the six tested microorganisms; in fact, Tp.6B was the only endophyte which positively affected the pathogenic fungal growth of Candida albicans ATCC 10231. Fungal endophyte of Tp.5F exhibited inhibitory actions against 3 tested microbial pathogens and it was observed amongst the highest suppressor for Bacillus subtilis ATCC 6633. Bacterial endophyte of Tp.1B significantly (P = 0.04) displayed antagonistic effect against the growth of 4 tested pathogens, where this endophyte was recorded as the highest inhibitor for Pseudomonas aeruginosa ATCC 9027 (Table 3).
Table 3.
Antimicrobial activities of microbial endophytes.
| Microbial isolates | Diameter of clear zone (mm) |
|||||
|---|---|---|---|---|---|---|
| P. aeruginosa | S. typhi | E. coli | S. aureus | B. subtilis | C. albicans | |
| Tp.1B | 16.7 ± 0.3a | 11.3 ± 0.3b | 8.3 ± 0.3b | 0c | 6.00.6b | 0b |
| Tp.2B | 0f | 8.6 ± 0.3c | 0c | 0c | 0d | 0b |
| Tp.3B | 13.7 ± 0.3b | 8.3 ± 0.3c | 0c | 0c | 0d | 0b |
| Tp.4B | 13.3 ± 0.8b | 0e | 0c | 0c | 3.7 ± 0.1c | 0b |
| Tp.5B | 12.7 ± 0.6b | 0e | 0c | 0c | 0d | 0b |
| Tp.6B | 16.6 ± 0.3a | 19.3 ± 0.7a | 15.3 ± 0.3a | 20.0 ± 1.1a | 15.7 ± 0.3a | 12.6 ± 0.3a |
| Tp.7B | 0f | 7.3 ± 0.4d | 0c | 0c | 0d | 0b |
| Tp.1F | 9.3 ± 0.4d | 0e | 0c | 0c | 0d | 0b |
| Tp.2F | 11.7 ± 0.4c | 0e | 0c | 0c | 7.0 ± 1.1b | 0b |
| Tp.3F | 8.7 ± d | 0e | 0c | 0c | 0d | 0b |
| Tp.4F | 7.3 ± e | 0e | 0c | 0c | 0d | 0b |
| Tp.5F | 13.3 ± 0.3b | 0e | 0c | 14.7 ± 0.3b | 14.6 ± 0-3a | 0b |
Tested microorganisms are, Gram positive bacteria: Staphylococcus aureus ATCC 6538 (S. aureus), Bacillus subtilis ATCC 6633 (B. subtilis); Gram negative bacteria: Escherichia coli ATCC 8739 (E. coli), Pseudomonas aeruginosa ATCC 9027 (P. aeruginosa) and Salmonella typhimurium ATCC 14028 (S. typhimurium); unicellular fungi: Candida albicans ATCC 10231 (C. albicans).
Values within the same column with different letters are significantly different (P ≤ 0.05) by LSD test, values are means ± SE (n = 6).
It was remarkable that isolated bacterial and fungal endophytes produced several extracellular enzymes; however, different endophytic isolates appeared variable enzymatic activities. Fungal isolates of Tp.5F and Tp.2F showed the maximum enzymatic activities compared to other endophytes (Table 4). Amongst bacterial endophytes, isolate of Tp.1B was recorded as the highest producer for amaylase (P ≤ 0.001) and cellulose enzymes (P = 0.04). The maximum bacterial production of pectinase and protease enzymes was found for bacterial endophytes of Tp.1B and Tp.6B (Table 4).
Table 4.
Extracellular enzymatic activities of microbial endophytes.
| Microbial isolates | Diameter of clear zones (mm) |
|||||
|---|---|---|---|---|---|---|
| Amylase | Pectinase | CMCase | Cellulase | Xylanase | Gelatinase | |
| Tp.1B | 20.7 ± 0.6a | 15.7 ± 0.3d | 25.3 ± 0.3d | 30.3 ± 0.3b | 25.3 ± 0.3c | 22.7 ± 0.3b |
| Tp.2B | 6.7 ± 0.3c | 12.7 ± 0.3f | 12.0 ± 0.5 g | 14.3 ± 0.3 g | 10.6 ± 0.8f | 17.7 ± 0.6c |
| Tp.3B | 7.0 ± 0.5c | 13.3 ± 0.8e | 13.0 ± 0.7f | 13.3 ± 0.9 g | 10.3 ± 0.9f | 14.0 ± 0.5d |
| Tp.4B | 6.3 ± 0.9c | 13 ± 0.5e | 10.3 ± 0.3 h | 14.0 ± 1.1 g | 11.7 ± 0.7f | 12.3 ± 0.3e |
| Tp.5B | 6.1 ± 0.6c | 13.3 ± 1.3e | 10.3 ± 0.9 h | 13.6 ± 0.7 g | 10.3 ± 0.9f | 17.7 ± 0.3c |
| Tp.6B | 9.3 ± 0.6b | 14.7 ± 0.7d | 15.3 ± 0.3e | 20 ± 1.2f | 14.7 ± 0.3e | 23.3 ± 2.4b |
| Tp.7B | 6.3 ± 0.3c | 12.6 ± 0.3f | 8.6 ± 0.3i | 13.7 ± 0.3 g | 11.0 ± 0.6f | 17.3 ± 0.3c |
| Tp.1F | 5.3 ± 0.3d | 31.0 ± 0.5b | 30.0 ± 0.6c | 28.6 ± 0.3c | 23.0 ± 0.5d | 24.6 ± 3.3b |
| Tp.2F | 0e | 36.7 ± 0.8a | 38.7 ± 0.7b | 34.7 ± 0.7a | 31.3 ± 0-7b | 34.7 ± 0.9a |
| Tp.3F | 5.4 ± 0.3d | 24.0 ± 0.6c | 25.0 ± 0.5d | 25.7 ± 0.6d | 22.3 ± 0.8d | 24.0 ± 0.6b |
| Tp.4F | 0e | 23.0 ± 1.2c | 26 ± 2d | 22.0 ± 0.5e | 22.4 ± 0.9d | 23.3 ± 0.3b |
| Tp.5F | 22.3 ± 1.5a | 37 ± 1a | 41 ± 1a | 36.3 ± 1.2a | 38 ± 1.2a | 33.3 ± 0.8a |
Values within the same column with different letters are significantly different (P ≤ 0.05) by LSD test, values are means ± SE (n = 6).
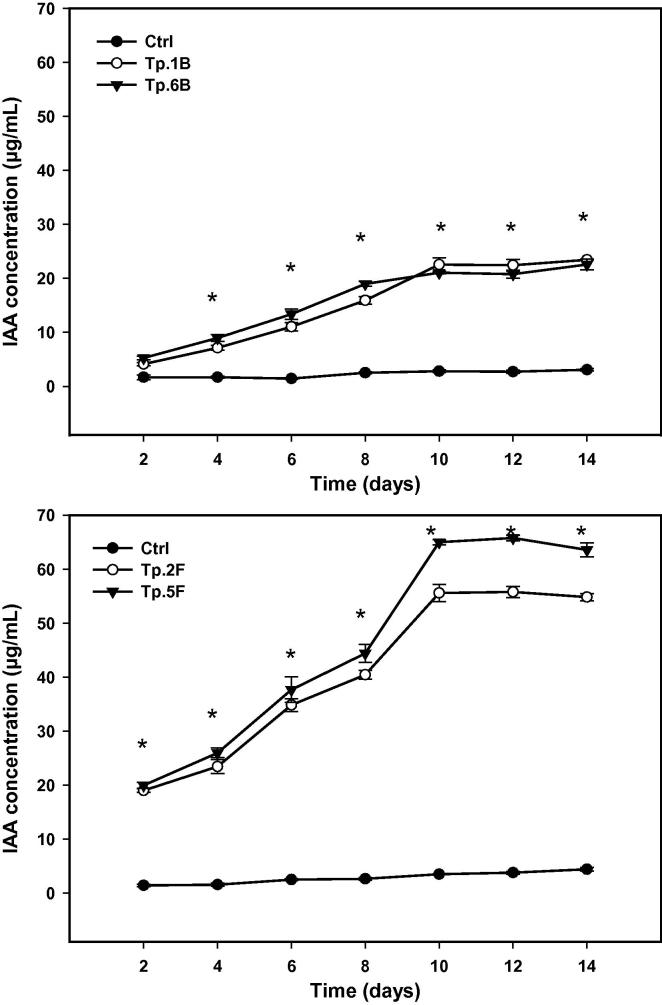
The results indicated that all endophytes tested produced IAA without or with tryptophan as precursor; however, it was observed that the range of IAA production increased with increasing tryptophan concentration in the media. Tryptophan concentration of 5 mg mL−1 induced the highest IAA amount produced by bacterial and fungal endophytes (Fig. 2). Fungal endophytes displayed higher IAA biosynthesis than those synthesized by bacterial endophytes. At all tryptophan concentrations or without tryptophan, fungal endophytes of Tp.5F and Tp.2F were the maximum IAA producers, where the highest bacterial IAA production was found for isolates of Tp.1B and Tp.6B. These four representative endophytes were further investigated for their ability of IAA biosynthesis at time courses of 2–14 days. Fungal endophyte of Tp.5F exhibited the highest IAA range of 19.9–63.5 µg mL−1 (P ≤ 0.001) while bacterial endophyte Tp.1B produced the maximum (P = 0.03) value (4.1–23.4 µg mL−1) of IAA (Fig. 3).
Fig. 3.
IAA production by the most potent of: (a) bacterial isolates (b) fungal isolates; in the presence of 5 mg/mL tryptophan and at different interval time course. At each time, asterisk denotes significant difference between control and endophytic isolates at significant level of (P ≤ 0.05) as determined by LSD test. Values are means ± SE (n = 6).
Effect of endophytes on plant growth
In order to investigate the effect of endophytes inoculation on plant growth, four representative endophytes of Tp.1B, Tp.6B, Tp.2F, and Tp.5F plus microbial consortiums of Mix.1-6B and Mix.2-5F were selected based on their potential PGP properties (Table 5, Table 6). Analysis of variance indicated that bacterial inoculation were significantly (F3,36 = 55.8; P ≤ 0.001) increased root length compared to the control (not inoculated seeds); however, inoculation with individual bacterial isolate or Mix.1-6B resulted in similar root length. Also, fungal inoculations caused significant (F3,36 = 59; P ≤ 0.001) increase the root length in contrast to non inoculated treatments. Regarding to the effect of endophytes on biomass production of maize in a greenhouse experiment, multi-comparison analyses revealed that the highest fresh (F3,20 = 315.8 and; P ≤ 0.001) and dry (F3,20 = 848.03 and; P ≤ 0.001) shoot weights were resulted from Tp.5F inoculation (Table 5). Moreover, Mix.2-5F–plant association led to a significant (F3,20 = 83.34 and; P ≤ 0.001) increase of fresh root weights comparable with not inoculated plants (Table 6). Remarkably, plants inoculated with fungal endophytes produced plant biomass higher than those observed for bacterial-inoculated plants.
Table 5.
Effect of bacterial inoculations on the growth properties of maize plants.
| Root length (cm) | Fresh weight (mg) |
Dry weight (mg) |
|||
|---|---|---|---|---|---|
| Bacterial treatments | Shoot | Root | Shoot | Root | |
| Control | 7.5 ± 0.85c | 535.8 ± 37.2b | 1011.6 ± 56.0b | 80.5 ± 3.2c | 222.3 ± 8.0c |
| Tp.1B | 11.5 ± 0.57a | 647.5 ± 22.2a | 1137.3 ± 30.8a | 129.1 ± 4.0 a | 328.3 ± 5.2a |
| Tp.6B | 10.45 ± 0.59a | 636.1 ± 16.0a | 1143.3 ± 18.6a | 110.7 ± 3.7b | 310.8 ± 4.8b |
| Mix.1-6B | 11.85 ± 1.2a | 643.0 ± 15.6a | 1169.8 ± 19.3a | 129.3 ± 6.9a | 332.0 ± 4.4a |
Values within the same column with different letters are significantly different (P ≤ 0.05) by LSD test, values are means ± SD (n = 6–10). Control is non bacterial inoculated plants; Mix.1-6B, bacterial consortium formed of a mix of bacterial isolates Tp.1B and Tp.6B.
Table 6.
Effect of fungal inoculations on the growth properties of maize plants.
| Root length (cm) | Fresh weight (mg) |
Dry weight (mg) |
|||
|---|---|---|---|---|---|
| Fungal treatments | Shoot | Root | Shoot | Root | |
| Control | 7.8 ± 0.78b | 559.5 ± 13.1a | 1012 ± 6.8d | 85 ± 3.2c | 222 ± 5.2c |
| Tp.2F | 12.05 ± 1.06a | 680.8 ± 8.4b | 1108 ± 44.7c | 133.1 ± 1.9b | 333.7 ± 16.7b |
| Tp.5F | 11.8 ± 1.13a | 732.3 ± 5.7a | 1225.8 ± 10.1b | 146.5 ± 1.8a | 346.2 ± 24.9a |
| Mix.2-5F | 12.8 ± 0.63a | 727.6 ± 14.6a | 1247.3 ± 36.0a | 147.8 ± 2.6a | 330.5 ± 8.4b |
Values within the same column with different letters are significantly different (P ≤ 0.05) by LSD test, values are means ± SD (n = 6–10). Control is non fungal inoculated plants; Mix.2-5F, fungal consortium formed of a mix of fungal isolates Tp.2F and Tp.5F.
Discussion
Medicinal plant of T. polium abundantly occurred in extremely arid area in Saint Katherine Protectorate of Egypt [12]. Bahramikia and Yazdanparast [13] reviewed 100 published works on phytochemical, pharmacological, and toxicological uses of extracts and active compounds isolated from T. polium during 40 years from 1970 to 2011. Antioxidant, anticancer, antibacterial, antifungal, and other biological activities have been reported for active compounds of T. polium, in particular terpenoids and flavonoids [13]. This plant could has different physiological and biological components that permit it to thrive in arid area with that valuable importance, and maybe some portion of its versatile adaptation would depend leastwise on its capacity to set up viable relationship with microbial endophytes. To our knowledge, this is the first report concerning the isolation of putative bacterial and fungal endophytes associated with this medicinal plant and characterization these endophytes as PGPE.
In the current study, seven bacterial and five fungal endophytes were isolated from medicinal plant of T. polium. Depending on PGP traits, four representative microbial isolates were selected and their potential role for PGP was determined. Genotypic characterization of bacterial isolates based on 16S rRNA sequences analysis showed that the isolated strains were classified in the genus Bacillus. The predominance of Bacillus genus as endophytic bacteria has been isolated from ginseng (Panax ginseng C.A. Meyer) [24], Lonicera japonica [25], and soybean (Glycine max L.) [26]. Hanna et al., [27] reported also the frequency of genus Bacillus in several perennial and annual plants found in north Sinai deserts, Egypt. Bacillus is one of the most commonly found as bacterial endophytes [28], [29]. Our results are in line with those reported that Bacillus populations have been widely found in various medicinal plants such as Glycyrrhiza spp., Pinellia ternate, Lycium chinense, Digitalis purpurae, Leonurus heterophyllus, Bletilla striata, Belamcanda chinases, P. pedatisecta, and Taxus yunnanensis [30], [31], [32].
Phylogentic analysis of fungal ITS sequences showed the taxonomic status of fungal endophytes to the genus Penicillium. Selim et al. [12] obtained 6 fungal endophytes namely Aspergillus niger, Alternaria alternate, Nigrospora sphaerica, Penicillium corylophilum, Penicillium chrysogenoum, and white sterile mycelia isolated from T. polium. Nicoletti et al. [33] reviewed the endophytism ubiquitous of penicillium genus in several woody plants; also, different endophytic penicillium species were isolated from coffee plants [34]. Broad screening techniques based on culture-dependent or independent methods to determine the endophyte-plant association is required in order to understand the specific relationship between plants and endophytes. In this direction, the determination of the plant-associated endophytes would be useful for the biotechnological applications of endophytes as biocontrol or plant growth-promoting agents.
Microbial endophytes directly promote plant growth through the production of plant hormones particularly IAA or phosphorus mobilization. Indirect plant growth promotion caused by endophytes includes antimicrobial activities and ammonia production or the synthesis of degrading enzymes which significantly inhibit pathogenic microorganisms.
‘In addition to the role of IAA in plant growth regulation, microbial IAA integrates symbiotic interaction between plants and microorganisms [8]. The results revealed that the highest IAA concentration ranges of 19.9–63.5 µg mL−1 and 4.1–23.4 µg mL−1 were registered for fungal endophyte of Tp.5F and bacterial endophyte Tp.1B, respectively; suggesting that the ability of these endophytes to stimulate root development and promote plant growth. It has been observed that root growth regulation is the main phenotypic character mediated by IAA [35], [36]; also, IAA produced by bacterial endophytes positively affected root development of tomato plants [37]. IAA has a crucial role in plant root formation and cell division stimulation even though under harsh environmental condition [36], [38]. Data analysis showed that increased IAA concentration significantly accomplished with increased tryptophan levels. Interestingly, the isolated endophytes had capacity of IAA synthesis without tryptophan supplementation which reflects the conditions inside plant tissues, where IAA synthesis occurs with free tryptophan levels. In fact, our results supposed that microbial endophytes obtained in this study, potentially regulate plant growth by means of IAA synthesis. In addition, different endophytic microbial species achieved variable capacity to produce IAA levels in a manner that differentially affected plant growth. Previously, it was observed that IAA produced by bacterial endophytes stimulate plant growth and increase root area and subsequently enhance nutrients uptake from the soil [39]. Dawwam et al. [40] showed that bacterial endophytes produced different amount of IAA ranged from 0.36 mg mL to 14.77 mg mL−1 and from 0.6 µg mL−1 to 10.73 µg mL−1. Three endophytic fungi of Penicillium chrysogenum, Alternaria alternate, and Sterile hyphae were isolated from Asclepias sinaica and produce approximately high IAA ranges of 100–160 µg mL−1 and exhibited a significant increase in root length of maize plants [20].
Microbial phosphate solubilization is a promising tool as biofertilizers application; the results showed that microbial endophytes of Tp.2F, Tp.5F, Tp.6B, and Tp1B displayed the highest indices for phosphate solubilization which is the main contributor for increased plant growth. Karagöz et al. [41] revealed that secretion of organic acids is one of the main mechanisms which enable bacteria to mobilize the insoluble phosphate. Another mechanism by which the phosphorus solubilizing bacteria help plants to access the P is through excretion of protons or enzymatic production that solubilize insoluble forms of P or mineralize organic phosphorus and render it available to the plants for uptake [42], [43]. Inoculation with endophytes increased phosphate solubilization and significantly improved plant growth [44]. On the other hand, microbial production of ammonia potentially provides plants with their demand of nitrogen and not only considers a mechanism for plant growth stimulation but also increases the plant defence against pathogens colonization. Frequent nitrogen input in the soil mainly increases the cost of crop production; therefore, production of ammonia by endophytes is a desirable trait for plant growth promotion and soil fertility [45].
Enzymatic and antimicrobial activities are indirect mechanisms exhibited by endophytes for plant growth promotion. The results demonstrated that all isolated endophytes produced cellulose, pectinase, xylanase, and protease enzymes. These enzymes are responsible for hydrolytic actions which enable endophytes to penetrate plant tissue and establish symbiotic relationship between endophytes and host plant. The enzymatic activities of endophytes provide their host plants with a protection against pathogenic microorganisms through cell wall hydrolysis of pathogens [46]. Although endophytes obtain their nutrients from plant by enzymes secretion, these extracellular enzymatic activities improve plant nutrition and imply in plant senescence by protein and polysaccharides degradation [47]. In another view regarding to biotechnology, amylolytic and proteolytic enzymes of endophytes are being investigated to improve industrial processes for polysaccharides and protein biodegradation [48].
Suppression of microbial growth by crude filtrate of endophytes was determined in order to investigate antimicrobial activity of endophytes [49]. Results showed that all isolated endophytes displayed significant inhibition zone against at least one of the tested microbial pathogens. Therefore, endophytes with PGP characters integrated with antimicrobial actions potentially useful for crop production. Our results suggest the ability of these endophytes as biocontrol agents to inhibit the growth of pathogenic microorganisms. For biotechnological application of endophytes as biocontrol, the selection of microbial inoculants in regard to their direct ability to promote plant growth is crucial. However, other mechanisms which enable microbial inoculants to establish mutualism with plants without severe any detrimental symptoms are necessary required [50].
Endophytes localize inside plant tissues, that close linkage facilitates mutualism between endophytes and plant. Since endophytes are acting as producers for several bioactive compounds through various mechanisms, they offer several benefits significantly affect plant growth. Advantageously, being endophytes colonize plant tissues with symptomless appearance; they compete with pathogens on the same habitat and positively influence plant health. In a greenhouse experiment, the endophytes inoculated plants displayed better shoot and root dry weights compared to uninoculated plants. In addition, the representative isolates significantly increased plant root length than found for uninoculated plants. Endophytes isolated from plant and their inoculation enhanced the growth of other plants was previously found, where bacterial endophytes isolated from Lonicera japonica improved the growth of wheat plants, respectively [25]. Using microbial consortium with different endophytic isolates which have various PGP characters can probably integrated together and improve plant growth and health by employment diverse mechanisms within different periods of the plant life cycle [45]. Obviously, putative endophytes isolated in this study exhibited multiple routes to improve plant growth; moreover, better performance of inoculated plants grown in soil suggests the probable competition of isolated endophytes with soil microorganisms. However, neither this competition nor endophytic capacities of representative isolates in the tested plants have yet been proven in our study.
Conclusions
The present study revealed that medicinal plant of T. polium which naturally inhabitant arid conditions, is an ecological niche for diverse putative bacterial and fungal endophytes. These endophytes displayed various direct and indirect mechanisms for plant growth promoting without symptomatic injury; therefore, inoculation of maize plants with endophytic representative isolates stimulated plant growth and increased biomass production compared to uninoculated plants. This study suggests the potential application of these endophytes in agricultural traits could results in ameliorate plant production and health and in another way may lead to improve soil quality and fertility. However, further endophytes isolation and repetitive field experiments are required to support the current finding.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This work was conducted in: Microbial Biotechnology Laboratories, and Botanical Garden at Botany and Microbiology Department, Faculty of Science, AL-Azhar University. The author would like to thank Prof. Dr. Azab M. (Faculty of Science, AL-Azhar University) for his discussion and comments which greatly improved the manuscript.
Footnotes
Peer review under responsibility of Cairo University.
Reference
- 1.Bokhtiar S.M., Sakurai K. Effect of application of inorganic and organic fertilizers on growth, yield and quality of sugarcane. Sugar Tech. 2005;7(1):33–37. [Google Scholar]
- 2.Aktar M.W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A.L., Waqas M., Khan A.R., Hussain J., Kang S.-M., Gilani S.A. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World J Microbiol Biotechnol. 2013;29(11):2133–2144. doi: 10.1007/s11274-013-1378-1. [DOI] [PubMed] [Google Scholar]
- 4.Karthik C., Oves M., Thangabalu R., Sharma R., Santhosh S.B., Indra Arulselvi P. Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium(VI) toxicity. J Adv Res. 2016;7(6):839–850. doi: 10.1016/j.jare.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri A., Padda K.P., Chanway C.P. Seedling growth promotion and nitrogen fixation by a bacterial endophyte Paenibacillus polymyxa P2b–2R and its GFP derivative in corn in a long-term trial. Symbiosis. 2016;69(2):123–129. [Google Scholar]
- 6.Khan A.R., Ullah I., Waqas M., Shahzad R., Hong S.-J., Park G.-S. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J Microbiol Biotechnol. 2015;31(9):1461–1466. doi: 10.1007/s11274-015-1888-0. [DOI] [PubMed] [Google Scholar]
- 7.Murphy B.R., Doohan F.M., Hodkinson T.R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 2014;62(1):29–39. [Google Scholar]
- 8.Lin L., Xu X. Indole-3-acetic acid production by endophytic streptomyces sp. En-1 isolated from medicinal plants. Curr Microbiol. 2013;67(2):209–217. doi: 10.1007/s00284-013-0348-z. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka H., Akiyama M., Kobayashi K., Yamaji K. Fe and P solubilization under limiting conditions by bacteria isolated from Carex kobomugi Roots at the Hasaki Coast. Curr Microbiol. 2013;66(3):314–321. doi: 10.1007/s00284-012-0276-3. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y., Lou K., Li C. Growth promotion effects of the endophyte Acinetobacter johnsonii strain 3–1 on sugar beet. Symbiosis. 2011;54(3):159–166. [Google Scholar]
- 11.Malhadas C., Malheiro R., Pereira J.A., de Pinho P.G., Baptista P. Antimicrobial activity of endophytic fungi from olive tree leaves. World J Microbiol Biotechnol. 2017;33(3):46. doi: 10.1007/s11274-017-2216-7. [DOI] [PubMed] [Google Scholar]
- 12.Selim K.A., El-Beih A.A., AbdEl-Rahman T.M., El-Diwany A.I. Biodiversity and antimicrobial activity of endophytes associated with Egyptian medicinal plants. Mycosphere. 2011;2(6):669–678. [Google Scholar]
- 13.Bahramikia S., Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae) Phytother Res. 2012;26(11):1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S., Zhou N., Zhao Z.-Y., Zhang K., Wu G.-H., Tian C.-Y. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress. Curr Microbiol. 2016;73(4):574–581. doi: 10.1007/s00284-016-1096-7. [DOI] [PubMed] [Google Scholar]
- 15.Miller D.N., Bryant J.E., Madsen E.L., Ghiorse W.C. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol. 1999;65(11):4715–4724. doi: 10.1128/aem.65.11.4715-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic Acids Techniques in Bacterial Systematics. John Wiley & Sons; Chichester, United Kingdom: 1991. pp. 115–147. [Google Scholar]
- 17.White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press Inc; New York: 1990. pp. 315–322. [Google Scholar]
- 18.Jasim B., John Jimtha C., Jyothis M., Radhakrishnan E.K. Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul. 2013;71(1):1–11. [Google Scholar]
- 19.Singh P., Kumar V., Agrawal S. Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol. 2014;2014:7. doi: 10.1155/2014/426483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouda A.H., Hassan S.E.D., Eid A.M., Ewais E.E.D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.) Ann Agric Sci. 2015;60(1):95–104. [Google Scholar]
- 21.Lv Y.L., Zhang F.S., Chen J., Cui J.L., Xing Y.M., Li X.D. Diversity and antimicrobial activity of endophytic fungi associated with the alpine plant Saussurea involucrata. Biol Pharm Bull. 2010;33(8):1300–1306. doi: 10.1248/bpb.33.1300. [DOI] [PubMed] [Google Scholar]
- 22.Glickmann E., Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waqas M., Khan A.L., Hamayun M., Shahzad R., Kang S.-M., Kim J.-G. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Interact. 2015;10(1):280–287. [Google Scholar]
- 24.Vendan R.T., Yu Y.J., Lee S.H., Rhee Y.H. Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J Microbiol. 2010;48(5):559–565. doi: 10.1007/s12275-010-0082-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., Xu Y., Lai X.-H., Shan C., Deng Z., Ji Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz J Microbiol. 2015;46:977–989. doi: 10.1590/S1517-838246420140024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y.Z., Chen W.F., Li M., Sui X.H., Liu H.C., Zhang X.X. Bacillus endoradicis sp. nov., an endophytic bacterium isolated from soybean root. Int J Syst Evol Microbiol. 2012;62(Pt 2):359–363. doi: 10.1099/ijs.0.028936-0. [DOI] [PubMed] [Google Scholar]
- 27.Hanna A.L., Youssef H.H., Amer W.M., Monib M., Fayez M., Hegazi N.A. Diversity of bacteria nesting the plant cover of north Sinai deserts, Egypt. J Adv Res. 2013;4(1):13–26. doi: 10.1016/j.jare.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero F.M., Marina M., Pieckenstain F.L. The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol Lett. 2014;351(2):187–194. doi: 10.1111/1574-6968.12377. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y., Yang H., Zhang T., Sun J., Lou K. Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of Tianshan mountain. Appl Microbiol Biotechnol. 2014;98(14):6375–6385. doi: 10.1007/s00253-014-5720-9. [DOI] [PubMed] [Google Scholar]
- 30.Venieraki A., Dimou M., Pergalis P., Kefalogianni I., Chatzipavlidis I., Katinakis P. The genetic diversity of culturable nitrogen-fixing bacteria in the rhizosphere of wheat. Microb Ecol. 2011;61(2):277–285. doi: 10.1007/s00248-010-9747-x. [DOI] [PubMed] [Google Scholar]
- 31.Wulff E.G., van Vuurde J.W.L., Hockenhull J. The ability of the biological control agent Bacillus subtilis, strain BB, to colonise vegetable brassicas endophytically following seed inoculation. Plant Soil. 2003;255(2):463–474. [Google Scholar]
- 32.Miller K.I., Qing C., Sze D.M.-Y., Roufogalis B.D., Neilan B.A. Culturable endophytes of medicinal plants and the genetic basis for their bioactivity. Microb Ecol. 2012;64(2):431–449. doi: 10.1007/s00248-012-0044-8. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti R., Fiorentino A., Scognamiglio M. Endophytism of penicillium species in woody plants. Open Mycol J. 2014;8:1–26. [Google Scholar]
- 34.Vega F.E., Posada F., Peterson S.W., Gianfagna T.J., Chaves F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia. 2006;98(1):31–42. doi: 10.3852/mycologia.98.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Taghavi S., Garafola C., Monchy S., Newman L., Hoffman A., Weyens N. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol. 2009;75(3):748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overvoorde P., Fukaki H., Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol. 2010;2(6):a001537. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbamondi G.R., Tommonaro G., Weyens N., Thijs S., Sillen W., Gkorezis P. Plant growth-promoting effects of rhizospheric and endophytic bacteria associated with different tomato cultivars and new tomato hybrids. Chem Biol Technol Agric. 2016;3(1):1. [Google Scholar]
- 38.Bianco C., Imperlini E., Defez R. Legumes like more IAA. Plant Signal Behav. 2009;4(8):763–765. doi: 10.4161/psb.4.8.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobbelaere S., Vanderleyden J., Okon Y. Plant growth-promoting effects of Diazotrophs in the Rhizosphere. Crit Rev Plant Sci. 2003;22(2):107–149. [Google Scholar]
- 40.Dawwam G.E., Elbeltagy A., Emara H.M., Abbas I.H., Hassan M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann of Agric Sci. 2013;58(2):195–201. [Google Scholar]
- 41.Karagöz K, Ateşb F, Karagöz H, Kotan R, ÇakmakÇi R. Characterization of plant growth-promoting traits of bacteria isolated from the rhizosphere of grapevine grown in alkaline and acidic soils. Eur J Soil Biol 2012 2012/6//; 50: 144–50.
- 42.Taktek S., St-Arnaud M., Piché Y., Fortin J.A., Antoun H. Igneous phosphate rock solubilization by biofilm-forming mycorrhizobacteria and hyphobacteria associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza. 2017;27(1):13–22. doi: 10.1007/s00572-016-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taktek S., Trépanier M., Servin P.M., St-Arnaud M., Piché Y., Fortin J.A. Trapping of phosphate solubilizing bacteria on hyphae of the arbuscular mycorrhizal fungus Rhizophagus irregularis DAOM 197198. Soil Biol Biochem. 2015;90:1–9. [Google Scholar]
- 44.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Geng X., Xie R., Fu L., Jiang J., Gao L. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol Biofuels. 2016;9(1):016–0592. doi: 10.1186/s13068-016-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi Y.W., Hodgkiss I.J., Hyde K.D. Enzyme production by endophytes of Brucea javanica. J Agric Tech. 2005;1 [Google Scholar]
- 48.Zaferanloo B., Virkar A., Mahon P.J., Palombo E.A. Endophytes from an Australian native plant are a promising source of industrially useful enzymes. World J Microbiol Biotechnol. 2013;29(2):335–345. doi: 10.1007/s11274-012-1187-y. [DOI] [PubMed] [Google Scholar]
- 49.Jinfeng E.C., Mohamad Rafi M.I., Chai Hoon K., Kok Lian H., Yoke Kqueen C. Analysis of chemical constituents, antimicrobial and anticancer activities of dichloromethane extracts of Sordariomycetes sp. endophytic fungi isolated from Strobilanthes crispus. World J Microbiol Biotechnol. 2017;33(1):5. doi: 10.1007/s11274-016-2175-4. [DOI] [PubMed] [Google Scholar]
- 50.Chow Y.Y., Rahman S., Ting A.S.Y. Understanding colonization and proliferation potential of endophytes and pathogen in planta via plating, polymerase chain reaction, and ergosterol assay. J Adv Res. 2017;8(1):13–21. doi: 10.1016/j.jare.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]