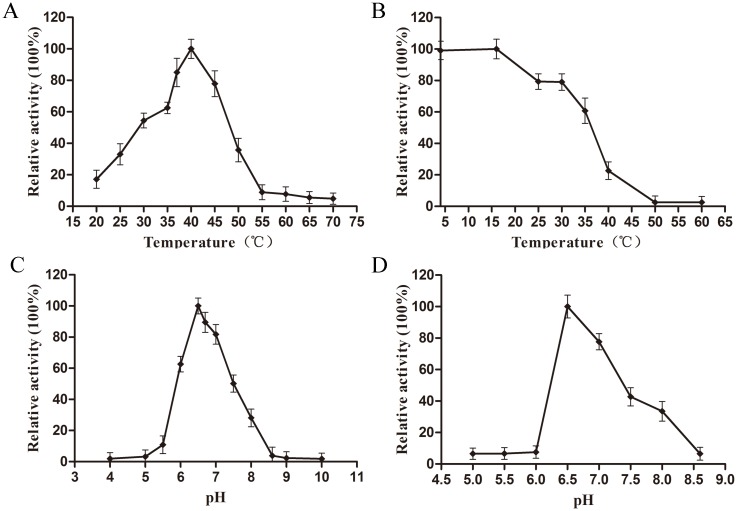
Fig 6. Effects of temperature and pH on the activity and stability of Ldc1E.
(A) Optimum reaction temperature of the recombinant Ldc1E. The enzyme activity was measured at various temperatures from 20°C to 60°C with 5°C intervals in 0.2 M Na2HPO4/0.1 M citric acid buffer (pH 6.5). Relative activity of 100% represents the specific activity of 1.50±0.06 U mg−1 protein. (B) Effect of temperature on the enzymatic activity of recombinant Ldc1E. Relative activity of 100% represents the specific activity of 1.53±0.06 U mg−1 protein. (C) Effect of pH on the enzymatic activity of recombinant Ldc1E. The enzyme activity was measured in 0.2 M Na2HPO4/0.1 M citric acid (4.0–8.0) and 0.1 M glycine–NaOH (8.0–10.0) at 40°C. Relative activity of 100% represents the specific activity of 1.53±0.06 U mg−1 protein. (D) Effect of stable pH on the enzymatic activity of the recombinant Ldc1E. Relative activity of 100% represents the specific activity of 0.93±0.05 U mg−1 protein.